Figure 5.
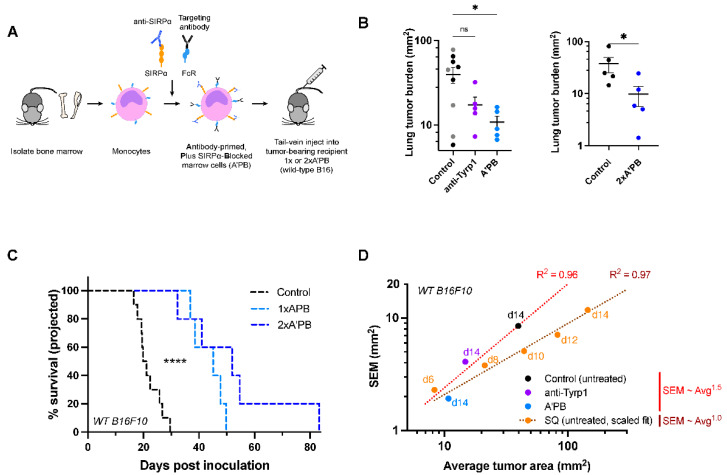
SIRPα-blocked, Tyrp1-targeted marrow cells suppress wild-type metastases, while anti-Tyrp1 treatment alone does not. (A) Schematic of SIRPα-blocked, Tyrp1-targeted (A’PB) fresh marrow cell production for tail-vein infusion into mice with WT B16F10 lung metastases. Monocytes and macrophage pre-cursors are the main marrow cells with SIRPα and Fc receptors, and the latter are pre-loaded with anti-Tyrp1 prior to infusion. Mice receive 2 × 107 antibody-engineered marrow cells intravenously on day 4 (1× A’PB) and 6 (2× A’PB). Additional anti-Tyrp1 was administered i.v. on days 4, 5, 7, 9, 11, and 13 (250 µg doses). (B) WT B16F10 tumor burden in mice treated with antibody-engineered marrow. Left: Control mice were treated with either isotype IgG2a antibody (gray, 250 µg dosing regimen) or received no treatment (black), while treated mice received either anti-Tyrp1 (250 µg dosing regimen) or A’PB marrow with anti-Tyrp1 (250 µg dosing regimen). Mean and s.e.m. (* p < 0.05, n.s. not significant, ordinary one-way ANOVA, n = 5 per group except isotype control n = 4). Right: Tumor burden in a separate experiment comparing control (untreated) or 2× A’PB groups. Lung tumor burden was assessed on day 14 post-inoculation. Mean and s.e.m. (* p < 0.05; this data is part of the experiment in Figure 7B, which describes the statistical test). (C) Projected survival advantages in A’PB-treated lung tumors. (n = 10 for control group, n = 5 per treatment group, **** < 0.0001, Log-rank test). (D) Variance of tumor areas scales with average tumor area of lung nodules, consistent with power law growth.