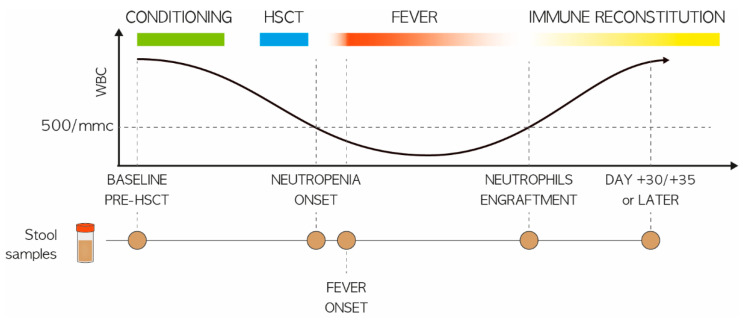
Figure 1.
Study design. Schematic representation of fecal sampling for pediatric patients undergoing HSCT, in relation to the development of FN. Circles indicate the sampling timepoints, i.e., at baseline (before transplant; PRE), at the onset of neutropenia (day −2/+2; P2), at the onset of fever (day +4/+5; P5), at engraftment (TAKE) and after engraftment (day +20/+30; P20). Patients were stratified based on the median of total fever days into two groups: (i) less than or equal to three days (n = 16) and (ii) more than three days (n = 21).