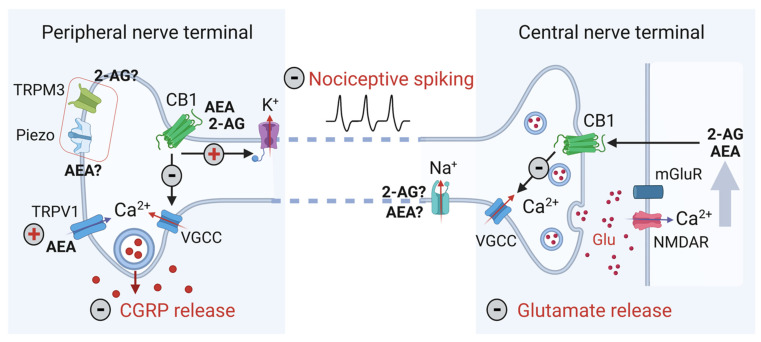
Figure 3.
EndoCBs interfere with multiple ion channels in peripheral migraine pain mechanisms. Nociceptive spiking, Ca2+-dependent CGRP release in the peripheral nerve terminal (left), and glutamate release in the central nerve terminal (right), are the main targets for endoCBs leading to pain inhibition. In the peripheral nerve terminal, the activation of CB1 receptors by endoCBs results in the inhibition of voltage gated calcium ion channels (VGCC), resulting in reduced CGRP release. The CB1-mediated opening of potassium ion channels reduces excitability and diminishes nociceptive spiking. AEA also acts as a direct agonist of TRPV1 receptors, thus opposing peripheral anti-nociception via CB1 mechanism. Peripheral terminals also express mechanosensitive TRPM3 and Piezo ion channels (in the red box), which can potentially be modulated by endoCBs through modifications of the lipidic environment. In the central nerve terminal, glutamate release stimulates endoCBs synthesis by postsynaptic Ca2+ influx through NMDA receptor and PLC enhancement following mGluR activation. EndoCBs retrogradely approaching presynaptic terminals reduce glutamate release by blocking VGCC. The action of endoCBs is mediated by CB1 receptors but they can also work as allosteric modulators, directly targeting sodium ion channels and thus, further affecting the generation and propagation of nociceptive spikes. Plus (+) and minus (−) symbols indicate the enhancement or inhibition of ion channels by endoCBs, respectively.