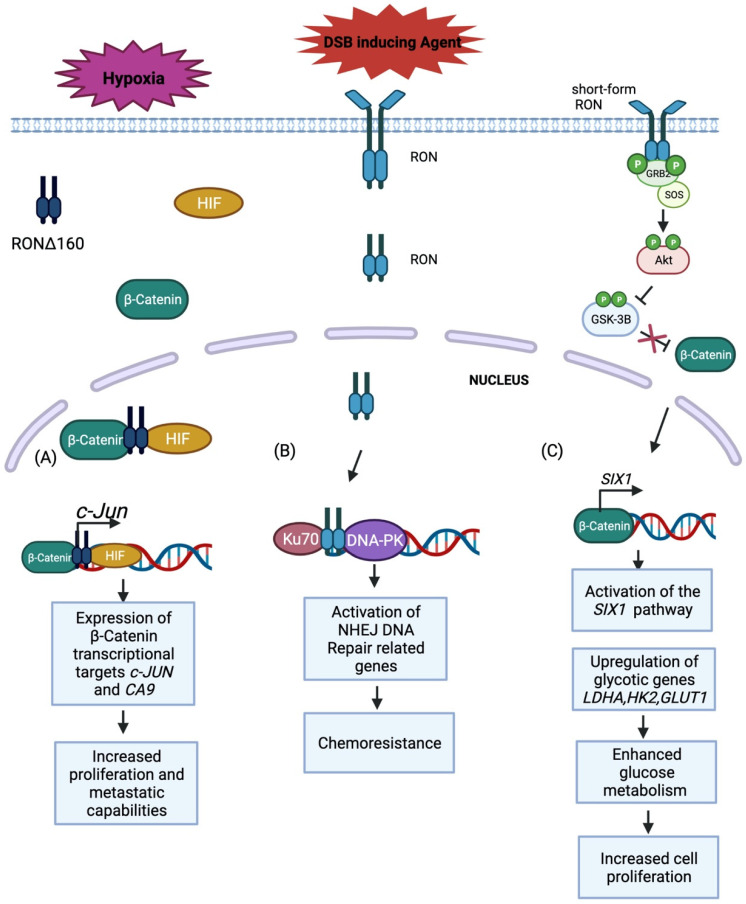
Figure 2.
Model demonstrating the role of RON as a transcription factor that promotes cell survival during cellular stress. (A) Under hypoxic conditions, a RON splicing variant, RONd160, binds to hypoxia induced factor (HIF) and b-catenin to form a complex that translocates to the nucleus and drives the expression of b-catenin target genes like c-jun and ca-9. The upregulation of these genes leads to increased cell proliferation and metastatic capabilities that drives tumorigenesis. (B) In response to treatment with chemotherapeutic agents, RON translocates to the nucleus and binds with Ku70 and DNA-PK to form a complex that drives the expression of genes related to Non-homologous endjoining (NHEJ) pathways. This form of dna repair prevents apoptotic events that would normally be activated due to DSB in DNA, making these cells resistant to chemotherapy. (C) A constitutively active form of RON (sf-RON) activates the AKT pathway through phosphorylation. AKT then phosphorylates GSK-3B to inhibit its function. Inactive GSK-3B cannot inhibit B-catenin which is free to enter the nucleus and activate the S1X1 pathway which the drives the expression of glycotic genes. Upregulation of these genes enhances glucose metabolism which increases cell proliferation that is necessary for tumorigenesis.