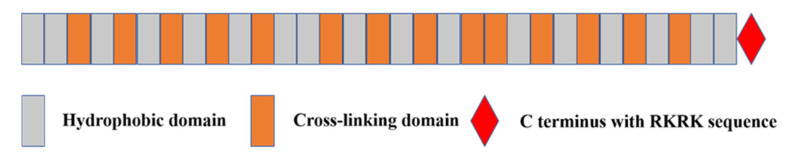
Figure 2.
Domain structure of tropoelastin. Valine, proline and glycine-rich hydrophobic domains are involved in the self-assembly or coacervation of tropoelastin. Hydrophilic domains, rich in lysine, alanine and proline residues are arranged alternately between hydrophobic domains and contribute to the cross-linking of tropoelastin. The C-terminal RKRK motif binds with integrins to regulate cell adhesion and interacts with microfibrils to facilitate elastic fibre assembly.