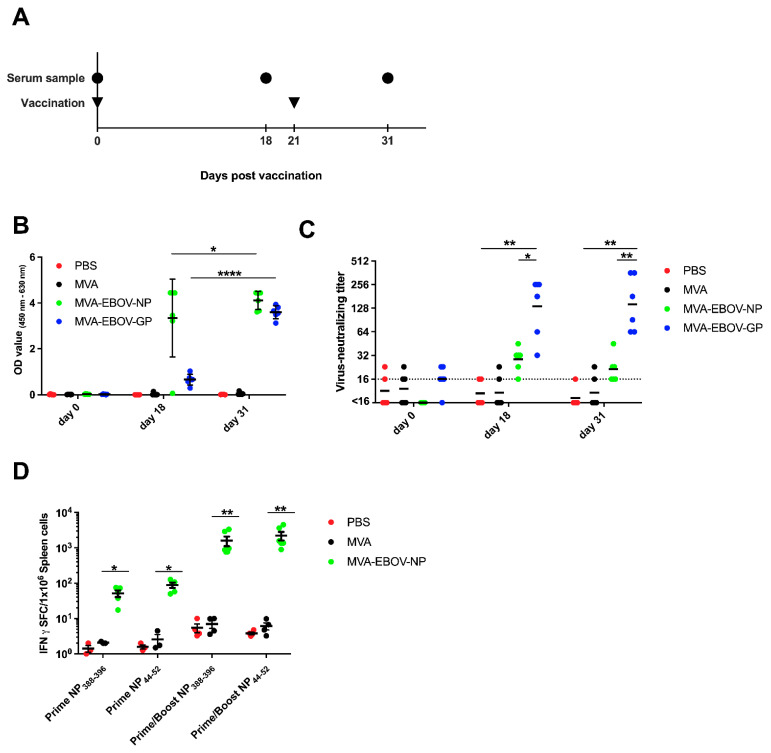
Figure 2.
Immunogenicity of recombinant MVA-EBOV vaccines. (A) IFNAR-/- mice were vaccinated intramuscularly with either PBS or 108 PFU non-recombinant MVA (MVA) as controls or with 108 PFU of the recombinant viruses MVA-EBOV-NP and MVA-EBOV-GP in a prime-boost regimen. Serum samples were taken at the indicated time points. (B) EBOV-virion ELISA performed with serum samples obtained at days 0, 18 and 31 after the prime vaccination of mock-vaccinated and vaccinated mice. Mean optical density values were measured at 450–620 nm; error bars: standard deviation; PBS: n = 5 mice, MVA, MVA-EBOV-GP, MVA-EBOV-NP: n = 6 mice. (C) Geometric mean EBOV-neutralizing titer determined by virus neutralization assay on days 0, 18 and 31 after prime vaccination, respectively; dotted line: limit of detection; PBS: n = 5 mice, MVA, MVA-EBOV-GP, MVA-EBOV-NP: n = 6 mice. (D) EBOV NP-specific CD8+ T cell response was measured by ELISPOT assay. Splenocytes were prepared at eight days after prime or prime/boost vaccination. NP388–396 or NP44–52 epitope-specific, IFN-spot forming CD8+ T cells (IFN-SFC) were quantified; bar: mean, error bars: standard error of the mean; PBS, MVA-prime NP: n = 3 mice; PBS, MVA-prime/boost NP: n = 4 mice; MVA-EBOV-NP-prime NP: n = 5 mice; MVA-EBOV-NP-prime/boost: n = 6 mice. * p < 0.05; ** p < 0.01, **** p < 0.0001.