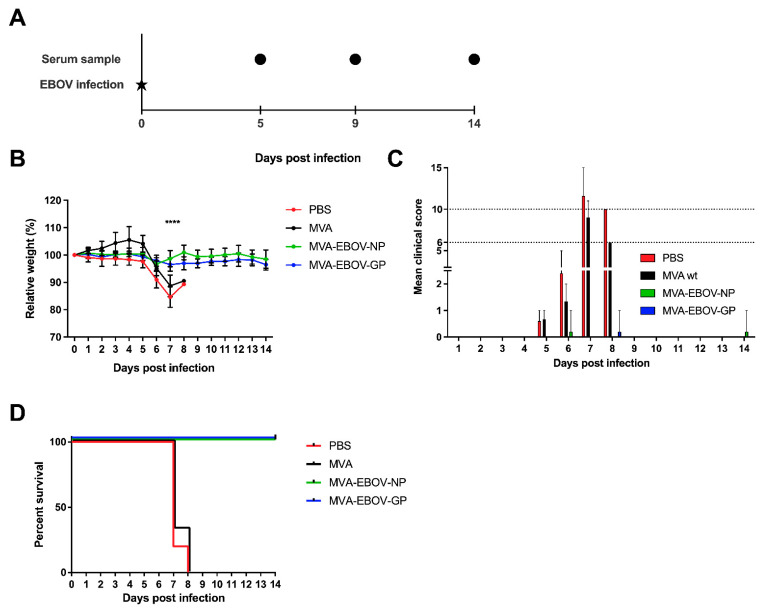
Figure 3.
MVA-EBOV protection against EBOV infection. (A) Vaccinated IFNAR-/- mice were challenged by intranasal inoculation with 1000 PFU EBOV (Mayinga isolate) 65 days after primary vaccination; PBS, MVA-EBOV-GP, MVA-EBOV-NP: n = 5 mice, MVA: n = 3 mice. Serum samples were obtained at the indicated time points post EBOV infection. (B) Mean relative body weight changes post challenge; error bars: standard deviation; **** p < 0.0001. (C) Mean clinical score comprising body weight, general condition and spontaneous behavior (see Material and Methods; Table S1). The clinical end point was defined as an individual score of 10 or of 6 on two consecutive days (dotted lines); error bars: maximum score. (D) Kaplan–Meier survival curves.