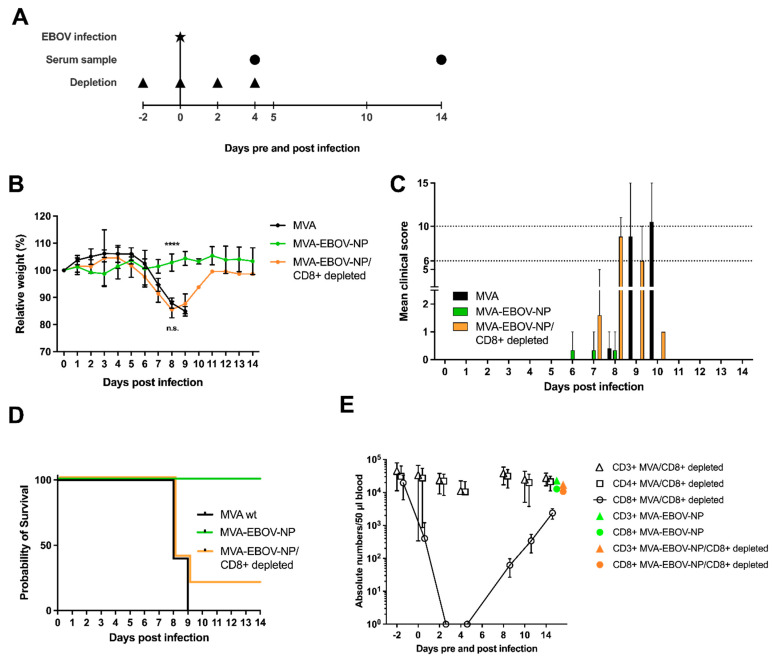
Figure 6.
CD8+ T cells are required for protective MVA-EBOV-NP immunization. (A) IFNAR-/- mice were vaccinated as described above with either 108 PFU non-recombinant MVA (MVA) as control or with 108 PFU of the MVA-EBOV-NP; MVA, MVA-EBOV-NP/CD8+ depleted: n = 6 mice, MVA-EBOV-NP: n = 3 mice. Depletion of CD8+ T cell was carried out by intraperitoneal injection of an anti-CD8+ antibody at the indicated time points. MVA-and MVA-EBOV-NP-vaccinated mice, as well as MVA-EBOV-NP-vaccinated and depleted mice were challenged with EBOV at day 65 after primary immunization. Depleted MVA-vaccinated mice were used as controls. Serum samples were obtained at the indicated time points after infection. (B) Mean relative body weight changes post challenge; error bars: standard deviation; **** p < 0.0001. (C) Mean clinical score comprising body weight, general condition and spontaneous behavior. The clinical end point was defined as an individual score of 10 or of 6 on two consecutive days (dotted lines); error bars: maximum score. (D) Kaplan–Meier survival curves. I FACS analysis for CD3+, CD4+ and CD8+ T cells. Numbers of the respective T cell population of MVA-vaccinated (CD8+ depleted) mice (n = 4) compared to one MVA-EBOV-NP-vaccinated (not CD8+ depleted) mouse and one surviving MVA-EBOV-NP-vaccinated (CD8+ depleted) mouse (data summarized in one graph for better visualization). 14 dpi numbers of CD8+ T cells of MVA-EBOV-NP-vaccinated (not CD8+ depleted) and surviving MVA-EBOV-NP-vaccinated (CD8+ depleted) mouse are comparable to non-infected control mice.