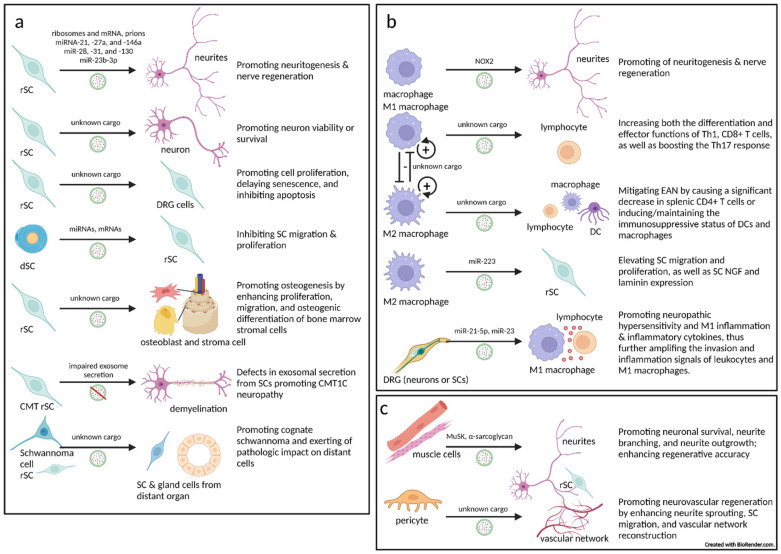
Figure 3.
The role and mechanisms of EVs in peripheral nerve physiology, inflammation, injury, and regeneration: (a) Schwann cell-derived EVs (SC-EVs) have been reported to promote neuritogenesis/neuroregeneration and neuronal survival/viability; exosomal secretion defects by SCs have been shown to involve the pathogenesis of inherited demyelinating neuropathy; SC-EVs also regulate SC proliferation, viability, and migration in an autocrine manner; SC-EVs can regulate target/innervated cell proliferation and differentiation; and SC-EV functionality is largely dependent on SC phenotypes—repair (rSC) or differentiated (dSC); (b) Dependent upon different phenotypes (M1/M2), macrophage-associated EVs have been reported to promote neuritogenesis and neuroregeneration, to modulate the immunomicroenviornment, and to support SC functionality. Macrophages can also be regulated by neuron- or SC-derived EVs; (c) Innervated (target) tissue/cell-derived EVs can participate in intercellular crosstalk with neurons, axons, and SCs. In the case of cancer, under innervation, the tumor (target tissue) is involved in intercellular crosstalk with neurons, axons, and/or SCs. rSC: repair Schwann cell; dSC: differentiated Schwann cell; CMT: Charcot-Marie-Tooth (disease); EAN: experimental autoimmune neuritis (mouse model for Guillain-Barré syndrome); NOX2: NADPH oxidase 2 complexes; DC: dentritic cell; DRG: dorsal root ganglion.