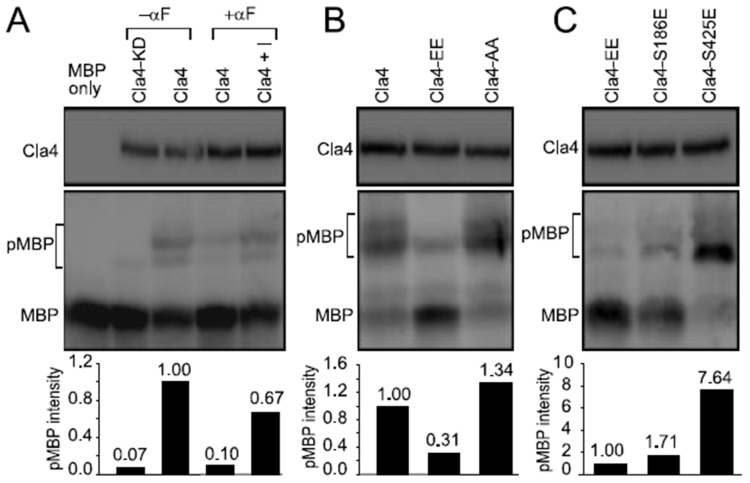
Figure 4.
In vitro phosphorylation of MBP by Cla4p. In vitro kinase assays were performed as described in the Materials and Methods. The intensity of the slower-migrating MBP was normalized by the level of immunoprecipitated Cla4p and expressed relative to wild-type Cla4p (A,B) or Cla4p-EE (C). pMBP indicates phosphorylated MBP. (A) Cla4p or Cla4p-KD in the fus3-as1 mutant was purified from mitotic cultures or cells treated with pheromone (MY15342 and 15343) for 2 h in the absence or presence of the inhibitor, 1-NA-PP1. (B) Wild-type, a phosphomimetic mutant Cla4p-EE (S186E, S425E), and the non-phosphorylatable mutant Cla4p-AA (S186A, S425A) were purified from mitotic extracts (MY15342, 15351, and 15647). (C) Cla4p single and double phosphomimetic mutants were purified from mitotic extracts (MY 15351, 15345, and 15349).