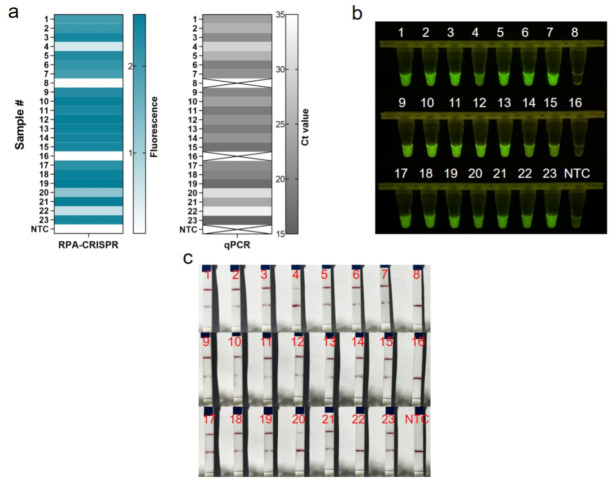
Figure 6.
Evaluation of RPA-CRISPR/Cas12a assay for MRSA with 23 clinical isolates. (a) Real-time fluorescence-based one-tube RPA-CRISPR/Cas12a detection of MRSA and selection of fluorescence of CRISPR reactions for 10 min for heat map, validated by qPCR in parallel. Samples 8 and 16 are negative, the rest are positive. ×: indicates a negative sample. NTC: no template control. (b) Endpoint visualization fluorescence-based one-tube RPA-CRISPR/Cas12a detection of MRSA. The only samples that do not show fluorescence signals are samples 8 and 16. (c) Detection of MRSA in clinical isolates with the RPA-CRISPR/Cas12a LFS assay. Test lines are visible on all samples except samples 8, 16 and 22, which do not show test lines.