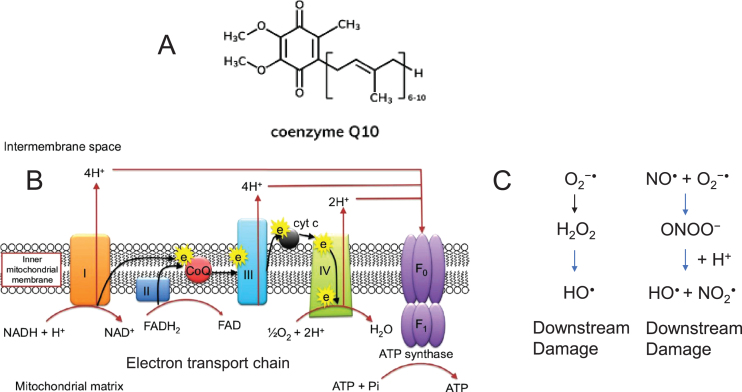
Fig. 2.
Schematic diagram of Coenzyme Q10 in the electron transport chain of the mitochondrial inner membrane. A) The structure of Coenzyme Q10, a lipid soluble component of the mitochondrial inner membrane. B) Mechanisms of oxidative stress involving mitochondria. The mitochondrial ETC reoxidizes reduced cofactors (NADH and FADH2) using molecular oxygen as the final electron acceptor, and the energy released in this process is captured in the form of ATP. The electron transport chain (ETC) depicting the position of Coenzyme Q10 (red) in the inner membrane. Coenzyme Q10 is critical for electron transport in the mitochondrial respiratory chain. The enzyme carries electrons from complexes I and II to complex III, thus participating in ATP production. I, II, III, IV, and V indicate protein components of the ETC. ‘e’ indicates electrons. and F1 are subunits of ATP synthase complex V (Complex V). ATP and ADP, adenosine triphosphate and diphosphate, respectively; CoQ 10, coenzyme Q10; FAD, flavin adenine dinucleotide; FADH, reduced flavin adenine dinucleotide. Image published with permission from Dove Medical Press, Rodick et al., Nutr Diet Supplem. 2017;10:1-11 [107]. C) Several components of the ETC chain (most often CI and CIII) generate O2•–. (left) The radical is converted into H2O2 by mitochondrial SOD. Through the Fenton reaction, H2O2 is converted into •OH, a molecule that produces oxidative cell injury through DNA damage, carboxylation of proteins, and lipid peroxidation. (right) •NO is produced by the activity of intracellular NOS. •NO can be combined with O2•– to produce peroxynitrite (ONOO–), a molecule that acts as a strong oxidant and can damage many cellular structures and alter their function. •NO, nitric oxide; •OH, hydroxyl radical; H2O2, hydrogen peroxide; O2•–, superoxide anion; ONOO–, peroxynitrite.