Fig. 3.
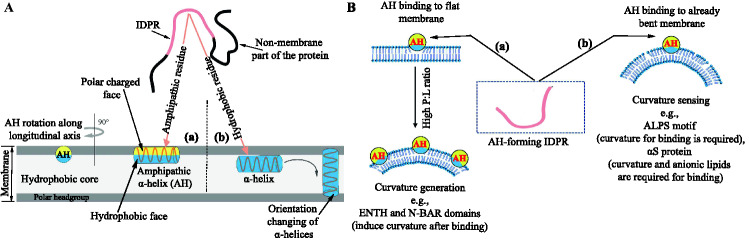
IDPR folding into alpha helices. A By folding-upon-binding transition, membrane-interacting IDPRs adopt amphipathic -helical (a), or only -helical structures (b). To find a better amphipathic orientation, AH is rotated along its longitudinal axis relative to its original conformation (leftmost). Reproduced from Cornish et al. (2020). Alpha helices are formed if helices are made of non-polar hydrophobic residues (b). Once helices are formed, they change the orientation and insert into the hydrophobic core of the membrane, referred to as TM helices. Next, TM helices are assembled into functional structures. The non-membrane part of the protein attached to the helix has not been shown in the schematic. Reproduced from White et al. (2001). B Lipid-binding mechanisms for AHs that sense and induce membrane curvature. (a) It is believed that proteins involved in curvature generation can bind to flat membranes by using hydrophobic and electrostatic interactions (specifically lipid for Epsin1). In high protein:lipid (P:L) ratios, the wedge effect, and the bilayer-couple mechanism cause curvature. (b) Some motifs, such as ALPS, require an already bent membrane for binding, as membrane insertion is driven only by hydrophobic interactions. In another example, the binding of S with its small and poorly hydrophobic residues and zwitterionic polar face is dependent on curvature and anionic lipids. Reproduced from Drin and Antonny (2010)