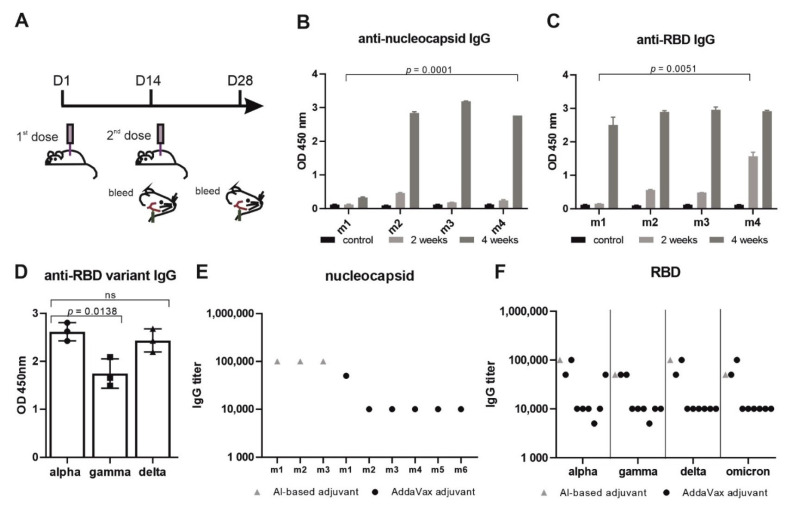
Figure 2.
‘VieVac’ produces prolonged immune responses against all known virus strains. (A) Adult C57BL/6J mice (n = 4) were immunized with 20 µg Imject™ Alum/VieVac (i.p.), with a booster injection carried out 14 days later. Blood sampling was performed 1 h before immunization and 28 days after the first immunization. (B) Anti-nucleocapsid and (C) anti-spike IgG responses were measured by ELISA upon administration of Imject™ Alum/’VieVac’ in 4 mice (m1, m2, m3, m4) 2 weeks (gray) or 4 weeks after the first injection (dark gray) as compared to controls (black). (D) Anti-RBD IgG cross-reactivity in mice (n = 3) against the alpha variant, relative to the gamma and delta variants treated with the prime-booster mode of Imject™ Alum/’VieVac’ 90 days prior. (E) Anti-nucleocapsid IgG end-titer in Imject™ Alum/VieVac (gray) and AddaVax™/’VieVac’ (black) prime-booster-immunized mice. (F) Anti-RBD IgG end-titer (alpha, gamma, delta, omicron variants) in Imject™ Alum/’VieVac’ (gray) and AddaVax™/’VieVac’ (black) prime-booster-immunized mice.