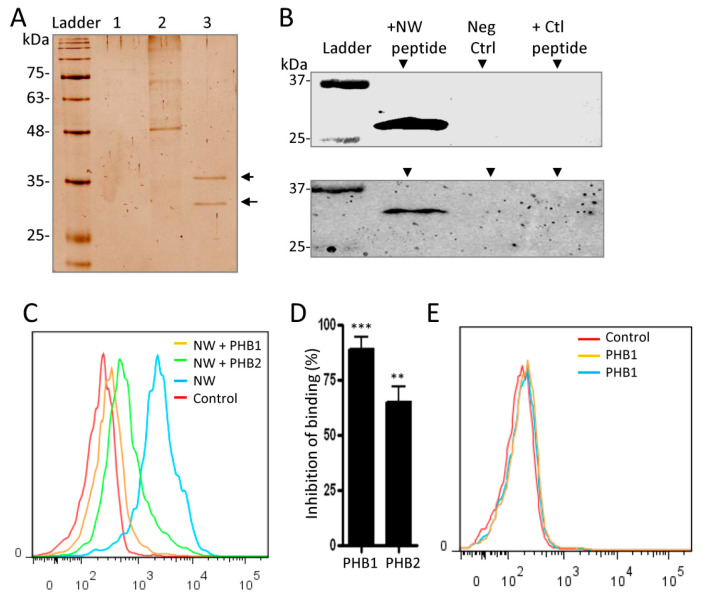
Figure 4.
NW peptide binds to prohibitins. (A) Proteins in whole-cell protein lysates were incubated with the biotinylated NW peptide or the control peptide, and peptide-binding proteins were pulled down by streptavidin magnetic beads and analyzed by SDS-PAGE followed by silver staining. Protein lysates incubated with only streptavidin-magnetic beads served as an additional control. Lane 1, proteins incubated with beads; Lane 2, proteins incubated with the control peptide; Lane 3, proteins incubated with the NW peptide. Two bands within the range of 35 to 25 kDa were detected in Lane 3. (B) Analysis of the pulled-down proteins by Western blotting. Experimental conditions are as in (A). Proteins were electrotransferred to nitrocellulose membranes and probed with a monoclonal antibody against PHB1 or PHB2 protein. Neg Ctrl, negative control (no peptide was added to the protein extracts). (C) Prohibitins inhibit the binding of the NW peptide to monocytes. The NW peptide (0.010 μg) was pre-incubated or not with prohibitins (3 μg each) for 30 min at RT before being added to monocytes and analyzed by flow cytometry. (D) Inhibition of peptide binding by prohibitins. Average values and SD of three independent experiments are shown. (E) Prohibitins did not show binding to monocytes. Monocytes incubated or not with prohibitins were washed and incubated with a PE-conjugated anti-His-tag monoclonal antibody to detect prohibitins by flow cytometry analysis. The data are representative of at least three independent experiments. ** p < 0.01; *** p < 0.005. PHB, Prohibitin; kDa, Kilodaltons.