Figure 3.
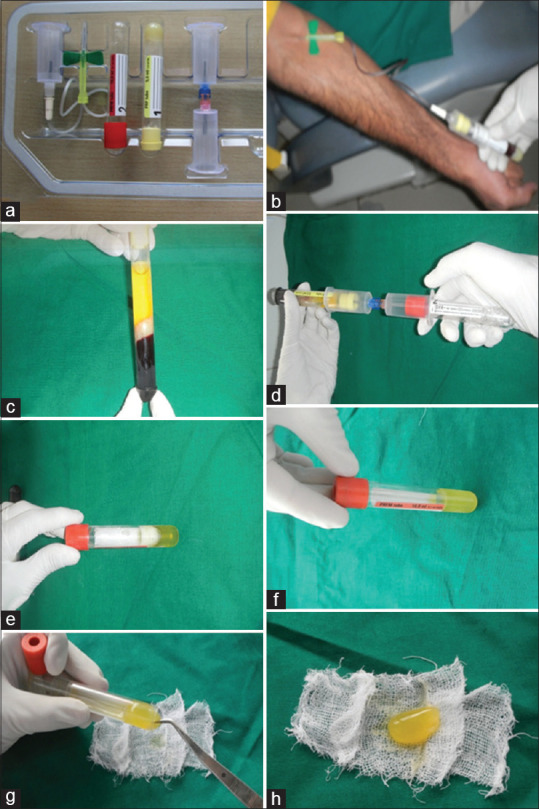
Kit and fabrication steps of platelet rich fibrin matrix. (a) Platelet-rich fibrin matrix kit. (b) Withdrawal of venous blood into a tube containing patented thixotropic separation gel. (c) Supernatant separated by centrifuge. (d) Transferring of supernatant to a second tube containing calcium chloride through a transferring device. (e) Transferred supernatant into the second tube for the second centrifuge. (f) Platelet-rich fibrin matrix procured. (g) Procured platelet-rich fibrin matrix gently being removed from the tube. (h) Final product platelet-rich fibrin matrix for application
