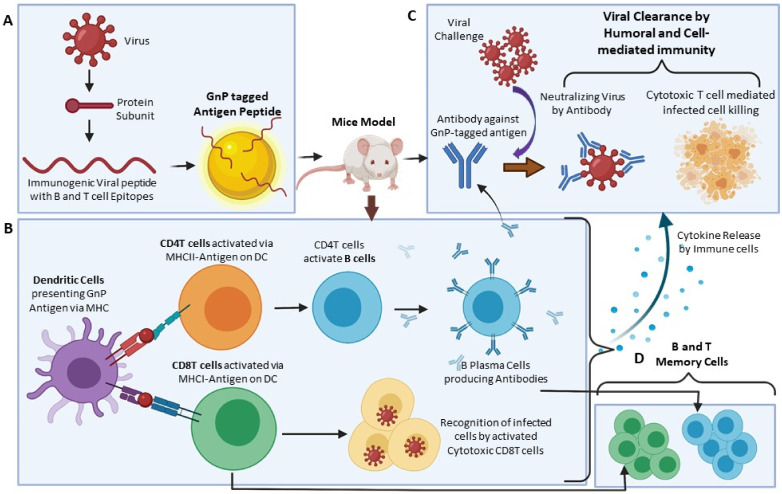
Figure 2.
A schematic representation of the use of GNPs in developing nanovaccines against a virus. (A) The protein subunit from the virus is isolated to determine the peptide sequence, which is both immunogenic for the host and conserved across multiple strains of the virus. The peptide is tagged with GNPs to create the novel nanovaccine and tested on the mice model. (B) The immune cells of the mice are triggered as the dendritic cells start presenting the peptides to the CD4 helper T cells and the CD8 cytotoxic T cells. The clonal expansion of the activated helper T cells and subsequent activation of the B cells into the plasma cells lead to the production of the antibodies specific to the peptide used for nanovaccine production. The cytotoxic T cells can recognize and deploy themselves in the killing of the infected cells. (C) The cytokines produced during the process of immune regulation of the nanovaccine produce a chemical milieu where the immune cells can favorably fight against the pathogens and shape the Th1 or Th2 immune response depending on the inflammation status. The antibodies can recognize the peptide sequence present in the whole virus and neutralize them effectively. (D) The B and T memory cells formed during this vaccination process can hold the information of the peptide used during the process and live long after. They are equipped to start an immediate immune response against any future challenge of the same virus and thus can eliminate them before they can cause major harm to the host.