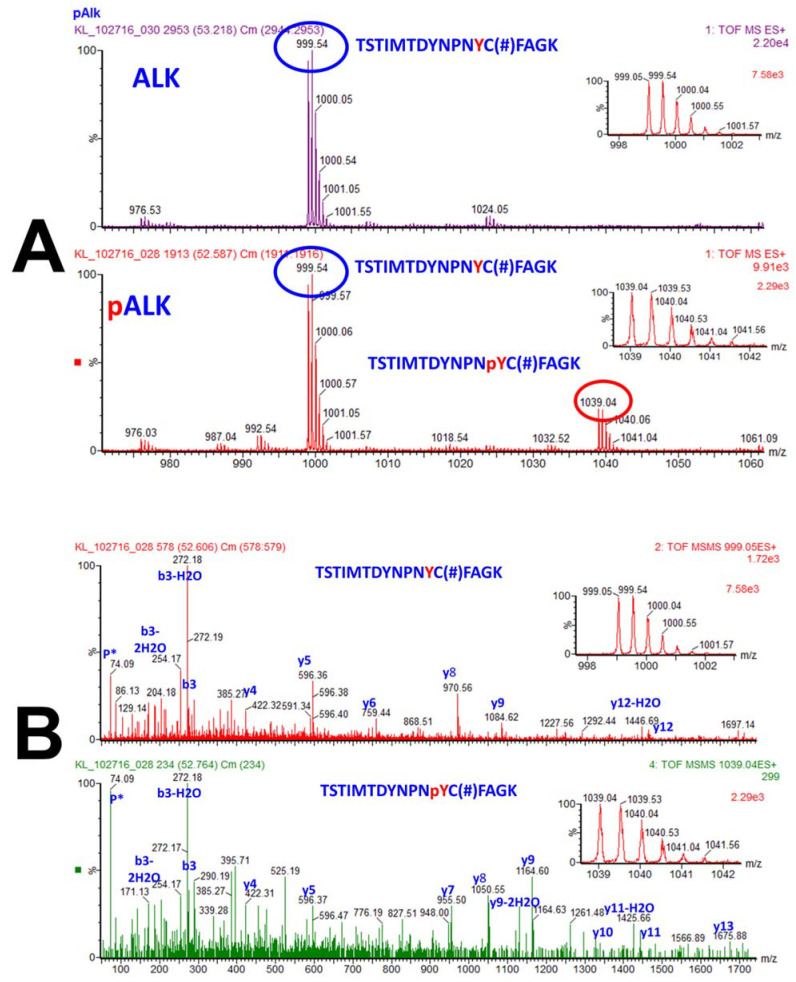
Figure 4.
Identification and quantification of a phosphopeptide, and localization of a phosphorylation on a tyrosine residue within a peptide. (A): The MS spectra of the precursor ions with m/z of 999.05 (2+) and 1039.04 (2+) that correspond to unphosphorylated peptide TSTIMTDYNPNYC(#)FAGK and its phosphorylated counterpart TSTIMTDYNPNpYC(#)FAGK. Note that C(#) represents cysteine modified by acrylamide (propionamide) and pY corresponds to phosphorylated tyrosine residue. While the top spectrum contains only the unphosphorylated peptide, the bottom spectrum contains precursor ions that correspond to both unphosphorylated and phosphorylated peptides (and the ratio of the two precursor ions indicates the percentage of phosphorylation of that peptide). Note that the peptide discussed is part of ALK, anaplastic lymphoma kinase (ALK) protein. (B): The MS/MS spectra of the precursor ions that correspond to the unphosphorylated and phosphorylated peptide not only confirm phosphorylation of this peptide, but also the location of this modification. Note that this peptide contains two tyrosine, two threonine and a serine residue, all of which can be potentially phosphorylated, yet the phosphorylation happens at only one specific tyrosine residue. Fragmentation of the precursor ions in MS/MS produced a series of b and y fragment ions that correspond to the unphosphorylated (top) and phosphorylated (bottom) peptide. Simply identifying y8 and y9 as not phosphorylated (top MS/MS spectrum), and y7, y8, and y9 are sufficient to demonstrate that the tyrosine residue adjacent and upstream of cysteine residue is the phosphorylated amino acid. Furthermore, y10 and y11 demonstrate that the next tyrosine upstream is not phosphorylated, and the m/z of the y13, b3, and the precursor ion indicate that none of the three serine and one threonine residues are phosphorylated. Reproduced from Reference [205].