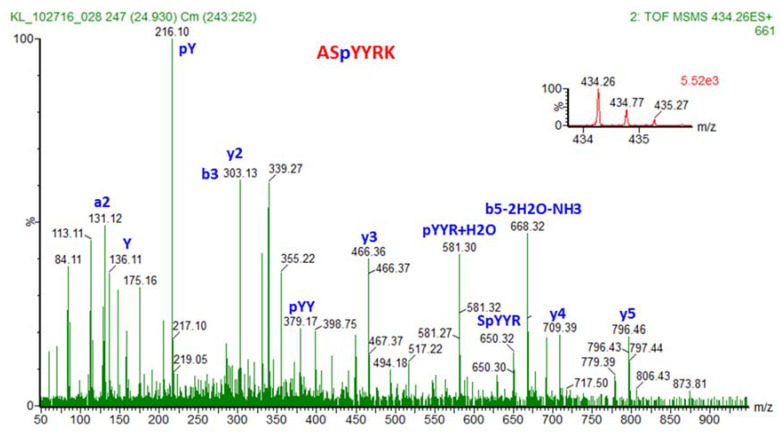
Figure 5.
MS spectrum of the precursor ion with m/z of 434.26 (2+), shown in the inbox figure, which corresponds to phosphorylated peptide ASpYYRK. Note that pY corresponds to phosphorylated tyrosine residue. Fragmentation of the precursor ion in MS/MS produced a series of fragment ions that correspond to the phosphorylated (bottom) peptide. From three potential amino acids that are phosphorylated (one serine and two tyrosine residues, all neighbors within the peptide sequence ASYYRK, only one tyrosine is phosphorylated, and not the second tyrosine, not the serine residues. This is clearly demonstrated by y3 ion, where tyrosine from the sequence YRK is not phosphorylated, y4 ion, where the second tyrosine from the sequence YYRK is phosphorylated (pYYRK) and y5 ion, where serine residue is not phosphorylated. The m/z of the precursor ion indicated that there is only one phosphate group on this peptide ASYYRK (namely ASpYYRK). Therefore, tandem mass spectrometry is highly specific in locating a specific modification within a peptide of a particular amino acid sequence. Reproduced from Reference [205].