Abstract
The photocatalytic activity of TiO2 can be enhanced by coupling it with other semiconductors and the semiconductor composites may find useful application in water treatment technologies. TiO2–Ag3PO4 composites were synthesized and characterized with XRD, SEM-EDX and DRS. The synthesized TiO2–Ag3PO4 showed high photocatalytic activity in the presence UV-vis light on rhodamine B, methylene blue and the pesticides imidacloprid, atrazine and pyrimethanil. LC-MS analysis of the photodegraded pyrimethanil led to the identification of hydroxylated and aliphatic derivatives of pyrimethanil. The photocatalytic activity of the coupled semiconductor was higher than that of the bare TiO2 and Ag3PO4 and this was attributed to the unique band matching between TiO2 and Ag3PO4 which resulted in efficient charge separation and subsequent reduction in the electron–hole recombination. In addition, the synthesized TiO2–Ag3PO4 showed strong adsorption for water soluble dyes implying that TiO2–Ag3PO4 can remove pollutants through photocatalysis and adsorption. The results from the study showed the potential application of TiO2–Ag3PO4 composite in water treatment technologies.
1. Introduction
Increased population and industrial activities have resulted in industrial effluent generation and pollution of water bodies, harmful to humans and aquatic life especially when they contain substances such as dyes, pesticides, heavy metals and pharmaceutical wastes.1
The world production of dyes has increased dramatically over the past years and these azo dyes, reactive dyes, solvent dyes for instance are used by the textile, food, adhesive, cosmetics, arts, construction, paints, glass and ceramic industries extensively.2 The release of these colored organic substances into our environment is a source of aesthetic pollution and is detrimental to the environmental ecosystems. In addition, large concentrations of organic dyes in the aquatic environment may be toxic to aquatic species and disulphonated azo dyes has been reported to be a major source of problem in drinking water plants globally.3
In addition there is high demand for pesticides globally as a result of increasing agricultural activities and the toxins in the pesticides are leached into surface and ground water sources which are detrimental to human health and aquatic life.4 The alarming impact of these activities on the environment has prompted the European Union to set standards under the European Directive 2000/60/EC that establishes maximum concentrations for pesticides and related harmful chemical substances in drinking waters to safeguard its detrimental effects on humans.5
High chemical stability and low biodegradability of these organic pollutants have necessitated the search for suitable treatment methods that can easily break down inherent chemical components. Techniques such as adsorption, biodegradation, ozonation and photo-Fenton processes have been adopted in treating dyes and pesticides pollutants.6 However, these methods have been reported to be ineffective in the treatment of polluted water bodies.7 Recent studies have been focused on the use of advance oxidation processes such as photocatalysis for the removal of dyes and pesticides from wastewaters,8 degradation of crude oil fractions,9 and for aquatic disinfection.10
Photocatalysis involves the generation of reactive oxygen species (ROS) in the presence of sunlight, semiconductor, dissolved oxygen and water. These ROS are able to oxidize and mineralize organic contaminants into carbon dioxide and inorganic anions.11 Hence, semiconductor based photocatalysts have received enormous attention because of their potential in solving some of the most challenging environmental problems. One of the most widely investigated photocatalyst is TiO2. Though TiO2 is chemically stable, its wide band gap energy limits its efficient utilization in sunlight irradiation. That is TiO2 is active in the UV region of the electromagnetic spectrum which makes up only ca. 4–5% of the electromagnetic spectrum and this limits its practical application. Developing semiconductor materials that are very sensitive to visible light and can be effectively utilized in practical applications has become the focus of many researchers. Different visible light active photocatalysts such as Au/TiO2,12 C–TiO2/g-C3N4,13 YFeO3,14 defect ZnO nanorods15 and ZnO/CuO16 have been developed by researchers. These semiconductor photocatalyst may be decorated with bimetallic nanoparticle cocatalyst to enhance their degradation efficiency.17 In addition, 3D graphene based photocatalyst have been developed and utilized in environmental pollutant degradation.18 Ag3PO4 with extremely high visible light photocatalytic activity has also been reported.19
Photocorrosion which involves the conversion of Ag+ to Ag0 in the absence of an electron acceptor has been reported as a major drawback of Ag3PO4 photocatalyst. This occurs as a result of the solubility of Ag3PO4 in aqueous solution.19a,20 In addition to using Ag3PO4 as a stand-alone photocatalyst, several composite photocatalyst have been developed by combining Ag3PO4 with AgI,21 gC3N4,22 carbon quantum dots,23 SrTiO3 (ref. 24) and TiO2.25 TiO2–Ag3PO4 has been reported to be the most efficient among the composite photocatalyst formed with Ag3PO4.26 A photocatalytic system made up of carbon fiber cloth as a porous substrate and Ag3PO4/TiO2 as a photocatalyst with a rhodamine B dye removal efficiency of ∼99.5% has been reported.27 Graphene oxide (GO) embedded ternary heterostructure nanocomposites of N–TiO2/Ag3PO4@GO with different weight ratios of N–TiO2 to Ag3PO4 has also been developed.28 It should however be mentioned that, to the best of the authors knowledge, efficiency of all the reported TiO2–Ag3PO4 photocatalyst was examined using water soluble dyes and phenols,29 and none of the articles reported a detailed adsorption kinetics and equilibrium studies. A detailed adsorption equilibrium and kinetic studies is required to understand the adsorption mechanism of Ag3PO4–TiO2 towards organic molecules.30
In this study, a one pot method was used to synthesize Ag3PO4–TiO2 photocatalyst. The synthesized photocatalyst was characterized with XRD, SEM-EDX, and DRS. The photodegradation efficiency was examined using rhodamine B dye, methylene blue dye and three pesticides; atrazine, imidacloprid and pyrimethanil. Detailed adsorption kinetic and equilibrium studies was conducted on the Ag3PO4–TiO2 using methylene blue dye. The photodegradation by-products obtained after subjecting pyrimethanil to photocatalysis were identified using LC-MS.
2. Experimental procedure
2.1. Materials
All chemical reagents used in this study were analytical grade and were purchased from Sigma Aldrich, U.K. The chemical reagents were used without further purification. Deionized water was used throughout this study.
2.2. Synthesis of Ag3PO4–TiO2 nanocomposite
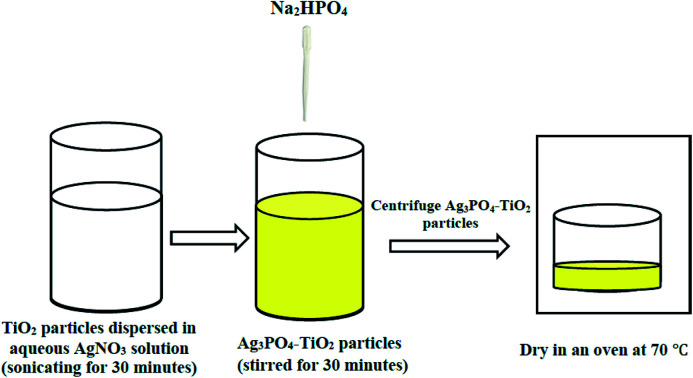
In a typical synthesis, a calculated amount of TiO2 (P25) was dispersed in 150 mL of 0.02 M AgNO3 by sonicating for 30 minutes. 122.5 mL of 0.2 M Na2HPO4 was then added dropwise while stirring. The resulting bright yellow solution was continuously stirred for 30 minutes and then centrifuged to collect the bright yellow precipitate. The collected precipitate was washed repeatedly (three cycles) with deionized water and dried at 70 °C to obtain Ag3PO4–TiO2 nanocomposite. Different amounts of TiO2 were used in the synthesis to obtain samples with 10 wt%, 25 wt%, 50 wt% and 75 wt% TiO2. A similar procedure was used to obtain Ag3PO4 but without the addition of TiO2. The synthesis scheme of the Ag3PO4–TiO2 nanocomposite is provided in Fig. 1 below.
Fig. 1. Synthesis scheme of the Ag3PO4–TiO2 nanocomposite.
2.3. Product characterization
2.3.1. X-ray diffraction (XRD)
The X-ray diffraction patterns were obtained with Bruker D8 advanced focus diffractometer fitted with position sensitive detector (LynxEye) and standard detector. Cu-Kα radiation (λ = 0.15405 nm) and a 2theta angular range of 10–80° were used.
2.3.2. Scanning electron microscopy/energy-dispersive X-ray spectroscopy
The morphology of the synthesized nanocomposites was examined with scanning electron microscopy (FEI Nova NanoSem) connected to EDX acquisition detector. The elemental composition was determined through EDX.
2.3.3. Diffuse reflectance spectroscopy (DRS)
DRS was conducted using Ocean Optics USB-4000 spectrometer with a dedicated reflectance probe. Illumination was supplied with a Deuterium/Halogen source across the UV-VIS range. The synthesized nanocomposites are compressed into a flat film between glass microscope slides prior to measurement and a commercial PTFE reflectance standard was sued as reflectance calibration.
2.3.4. Adsorption studies
The adsorption capability of the synthesized nanocomposite was examined using methylene blue dye. 100 mg of Ag3PO4–TiO2 was added to 200 mL aqueous solution of methylene blue (5 mg L−1). Aliquots of the methylene blue solution were taken at specified time intervals. The sampled solution was centrifuged at 6000 rpm for 5 minutes to remove the powdered nanocomposite. The concentration of methylene blue was obtained by measuring the absorbance at 655 nm wavelength using Ocean Optics 4000 USB modular UV-VIS spectrometer.
2.3.5. Photocatalytic methylene blue, rhodamine blue and pesticide degradation
The photocatalytic activity of the synthesized Ag3PO4–TiO2 was examined using rhodamine B (6 mg L−1) and methylene blue (8 mg L−1) dyes and pesticides (pyrimethanil, imidacloprid and atrazine). In a typical photocatalytic degradation of rhodamine B dye, 200 mL of rhodamine B solution in deionized water was used with a photocatalyst suspension concentration of 0.5 g L−1. A jacketed glass reactor with a quartz tube immersion well was used with illumination from 300 W Tungsten Halogen lamp. For each of the experiments, the solution was stirred in the dark for 30 minutes (24 hours for pesticide solutions) to attain an adsorption–desorption equilibrium for illumination. 2 mL aliquots were taken at specific time intervals and centrifuged at 6000 rpm for 5 minutes to remove the powdered photocatalyst. The dye concentration was quantified using Ocean Optics 4000 USB modular UV-vis spectrometer with an absorbance values taken at 554 nm. A similar procedure was used for the photocatalytic degradation of the methylene blue and pesticides. However, the absorbance values were taken at 665 nm for methylene blue, 269 nm (pyrimethanil), 270 nm (imidacloprid), and 222 nm (atrazine). The degraded pesticide solution obtained from pyrimethanil was analyzed with LC-MS to identify photodegraded products.
3. Results and discussion
3.1. Characterization of Ag3PO4–TiO2
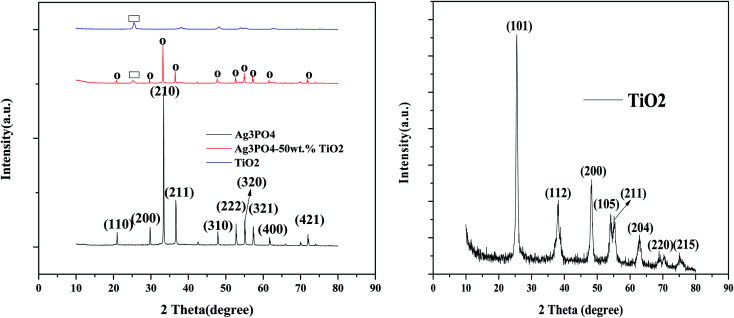
The XRD patterns of the pristine P25 TiO2, Ag3PO4, and Ag3PO4-50 wt% TiO2 are presented in Fig. 2. The XRD pattern for the Ag3PO4 showed well defined crystalline Ag3PO4 with sharp and intense diffraction peaks that can be indexed to the body-centered crystal structure (JCPDS no. 06-0505).
Fig. 2. XRD patterns for (A) Ag3PO4, TiO2 and Ag3PO4-50 wt% TiO2, (B) enlarged XRD pattern for TiO2 [O = Ag3PO4, ▭ = TiO2].
The dominant peaks at 33.4° and 36.6° correspond to the (210) and (211) crystallographic planes,31 respectively. On the other hand, TiO2 showed an intense peak at 25.5° which represents the (101) crystallographic plane and can be indexed to the anatase phase (JCPDS no. 21-1272). With the “rectangles” representing the anatase phase present in TiO2 and the circles representing the cubic structure of Ag3PO4, the prepared Ag3PO4–TiO2 composite has all the crystallographic planes present in the crystalline cubic structure of Ag3PO4 and the (101) plane of the anatase phase of TiO2. The as-prepared Ag3PO4–TiO2 is therefore expected to exhibit high photocatalytic activity. Though the Ag3PO4–TiO2 used for the analysis contained 50 wt% TiO2, the diffraction peaks of the TiO2 is weak. This can be ascribed to the relatively low crystallinity of TiO2 when compared with that of Ag3PO4.
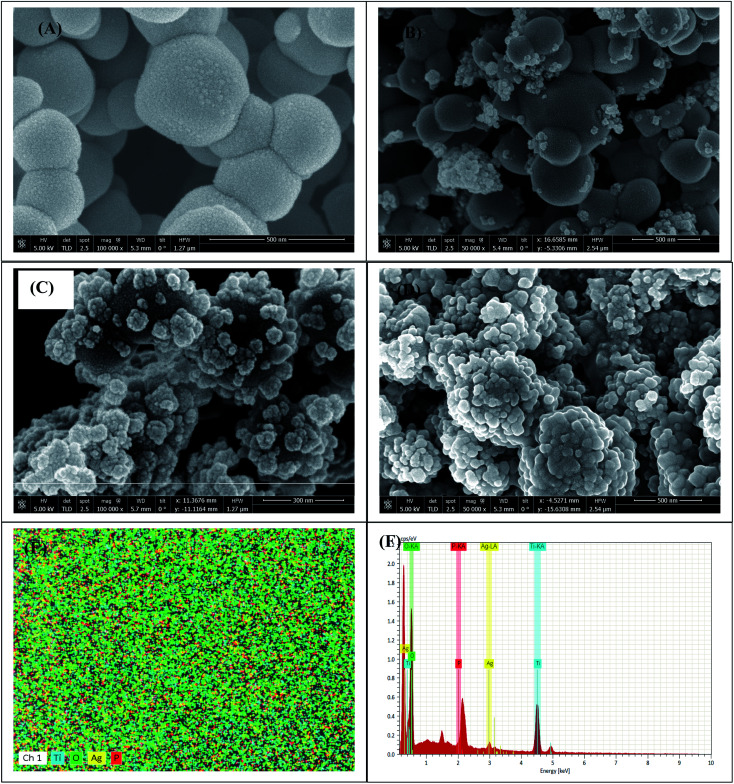
The morphology of the synthesized Ag3PO4 and Ag3PO4–TiO2 photocatalyst were examined with SEM and the images presented in Fig. 3. The pristine Ag3PO4 is irregularly shaped spherical particles with diameter of ca. 100–400 nm. The P25 used for this study has a much smaller diameter of ca. 20 nm. The SEM images showed the agglomeration of the TiO2 particles. The particle sizes of the Ag3PO4 and TiO2 in the Ag3PO4–TiO2 composite (Fig. 3 B–D) is similar to the pure Ag3PO4 and TiO2. It can be seen from Fig. 3B to D that the TiO2 covered the surface of the Ag3PO4 creating a heterojunction. The extent of the surface coverage is dependent on the wt% of the TiO2 used for the synthesis. This coverage is expected to influence the photocatalytic activity. Positively charged ions adsorbs strongly to the surface of TiO2.29a,32 Since the P25 TiO2 was first dispersed in AgNO3, the Ag+ ions adsorbed onto the surface of the TiO2. The reaction between the Ag+ and the PO43− occurred on the surface of the TiO2 when the Na2HPO4 was added, resulting in the formation of the TiO2–Ag3PO4 composite. The SEM-EDX (Fig. 3E and F) spectrum confirms the presence of Ti, O, Ag and P. The elemental mapping showed that the elements Ti and O were evenly distributed on the surface of the Ag3PO4.
Fig. 3. SEM images (A) Ag3PO4, (B) Ag3PO4-25 wt% TiO2, (C) Ag3PO4-50 wt% TiO2 and (D) Ag3PO4-75 wt% TiO2; (E) EDX mapping for Ag3PO4-50 wt% TiO2, (F) EDX spectrum for Ag3PO4-50 wt% TiO2.
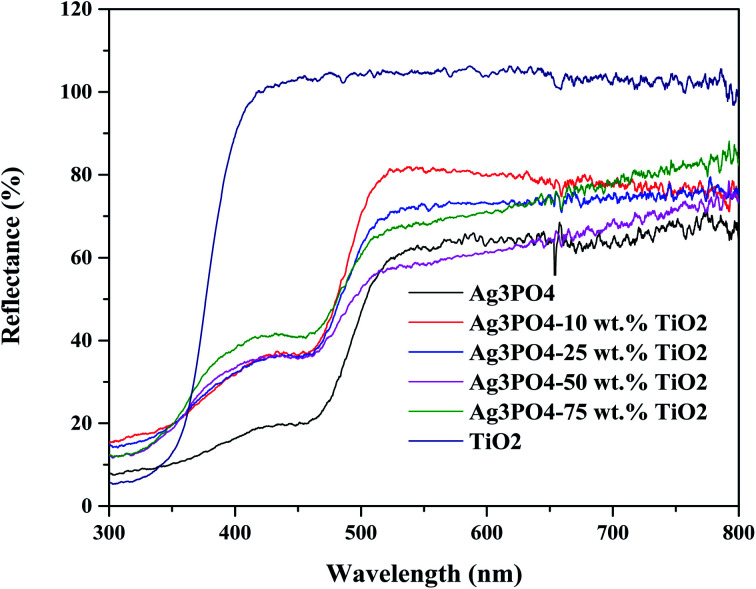
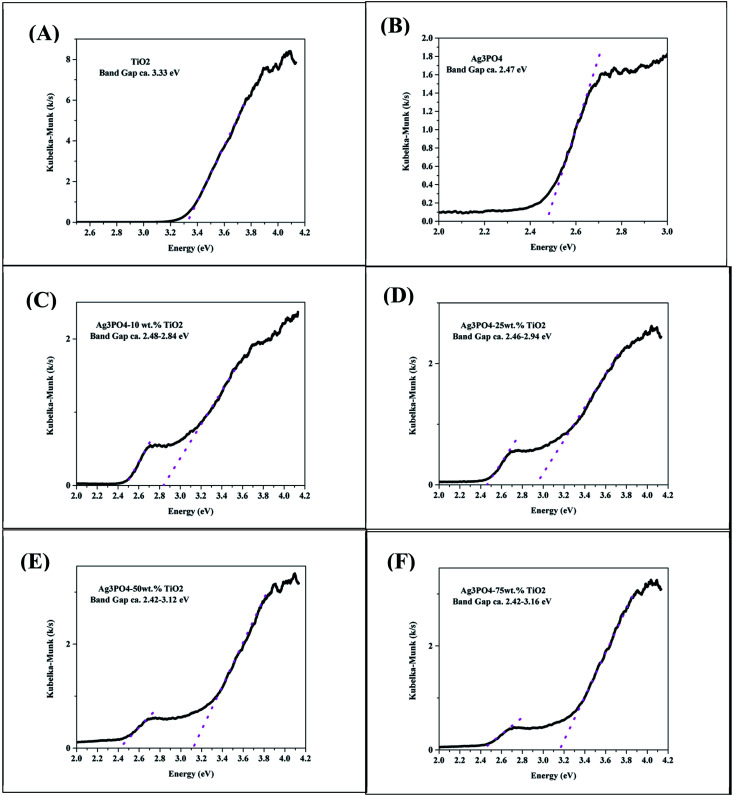
The UV-vis diffused reflectance spectra of the product with different wt% of TiO2 are shown in Fig. 4. The P25 TiO2 only absorbs light in the UV region of the electromagnetic spectrum with wavelength lower than approximately 390 nm. On the other hand, the pure Ag3PO4 has intense light absorption in the visible light region of the electromagnetic spectrum. The shape of the DRS spectra of the composite is a combination of that of Ag3PO4 and TiO2 further proving the absence of any chemical interaction between TiO2 and Ag3PO4. This agrees with observation made by Taheri et al.33 The composite Ag3PO4–TiO2 showed strong absorption in the visible light region due to the excellent visible light absorption by Ag3PO4. The composite Ag3PO4–TiO2 can therefore harvest more visible light and result in an improved photocatalytic activity. The optical band gap of the photocatalysts synthesized were estimated from the DRS spectra using the Kubelka–Munk model34 and are presented in Fig. 5. The optical band gap of the TiO2 and Ag3PO4 were estimated to be 3.33 and 2.47 eV, respectively. The composite samples are characterized by two absorption thresholds, one corresponding to the TiO2 and the other, Ag3PO4. A slight variation in the two absorption thresholds were observed upon varying the weight percent of the TiO2 used for the synthesis. The band gap for the absorption threshold corresponding to TiO2 decreased with decreasing wt% of TiO2 in the TiO2–Ag3PO4 composite as can be seen in Fig. 5.
Fig. 4. UV-vis DRS spectra of Ag3PO4, TiO2 and the various formulations of Ag3PO4–TiO2.
Fig. 5. Optical band gap estimation using Kubelka–Munk model for (A) TiO2, (B) Ag3PO4, (C) Ag3PO4-10 wt% TiO2, (D) Ag3PO4-25 wt% TiO2, (E) Ag3PO4-50 wt% TiO2 and (F) Ag3PO4-75 wt% TiO2.
3.2. Photodegradation of rhodamine B, methylene blue and pesticides
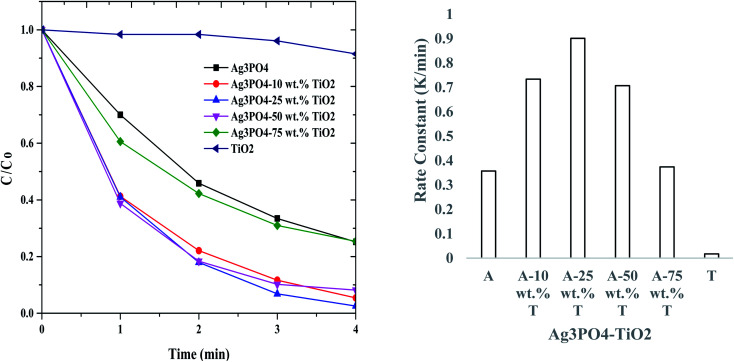
Fig. 6A shows the photocatalytic performance of rhodamine B solution (200 mL, 6 mg L−1) over Ag3PO4, TiO2 and Ag3PO4–TiO2 composites at a photocatalyst concentration of 0.5 g L−1 under UV-vis light irradiation. Before irradiation, the dye-photocatalyst solution was stirred in the dark for 30 minutes to achieve adsorption–desorption equilibrium. The percentages of rhodamine B adsorbed by Ag3PO4, TiO2 and Ag3PO4–TiO2 composite were 14.5, 18.8 and 12.0 wt%, respectively. The wt% of the rhodamine B adsorbed by the various formulations of the composite material was ca. 12%. The data obtained from the photocatalysis was fitted with a pseudo first order kinetics and the rate constants (k) calculated (Fig. 6B). Under UV-vis light irradiation, ca. 9.8% of rhodamine B (k = 0.0153 min−1) was degraded by TiO2 within the time (6 min) frame that the photocatalysis analysis was carried out. On the other hand, ca. 66% of the rhodamine concentration was degraded by Ag3PO4 within 6 min (k = 0.168 min−1). The rate at which the concentration of the dye solution degraded by the composite (Ag3PO4–TiO2) decreased was significantly higher than that of Ag3PO4 and TiO2. For the same time period (6 min), the degradation efficiency obtained from the composites were 90, 98, 96 and 79% for Ag3PO4-10 wt% TiO2 (k = 0.3571 min−1), Ag3PO4-25 wt% TiO2 (K = 0.5406 min−1), Ag3PO4-50 wt% TiO2 (K = 0.5069 min−1) and Ag3PO4-75 wt% TiO2 (K = 0.2438 min−1), respectively. These values indicate that Ag3PO4 and TiO2 act synergistically to enhance photodegradation. This is so because the degradation efficiency and the rate constants of the composite Ag3PO4–TiO2 were significantly higher than that of the individual components. The synergy was highest at 25 wt% TiO2. A similar observation was reported by Taheri et al.33 This observation can be attributed to the formation of a heterojunction between the TiO2 and the Ag3PO4.33 In addition, the photocatalytic activity is also affected by the extend of surface coverage. To obtain maximum photocatalytic activity, the extend at which the Ag3PO4 surface is covered by TiO2 should be minimized. The uncovered surface of Ag3PO4 is utilized for maximum absorption of visible light.
Fig. 6. (A) Photocatalytic activities of Ag3PO4, TiO2 and Ag3PO4–TiO2 (10, 25, 50, and 75 wt% of TiO2) and (B) rate constants of Ag3PO4(A), TiO2 (T) and Ag3PO4–TiO2 (A-wt% T) on 6 mg L−1 rhodamine B dye.
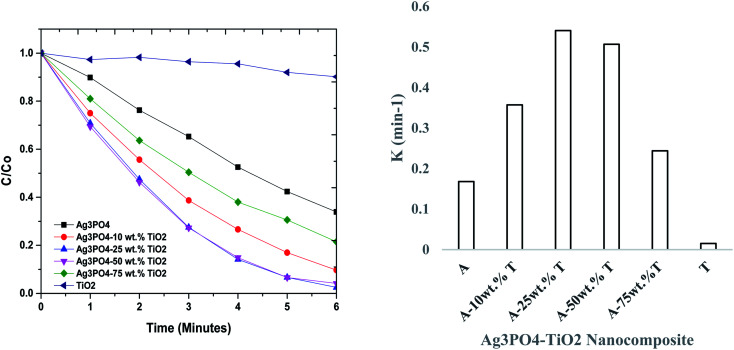
The degradation of methylene blue (200 mL, 8 mg L−1, 0.5 g L−1 catalyst concentration) was also examined under UV-vis light irradiation and the results shown in Fig. 7. After the adsorption–desorption equilibrium was attained, 8.9 (Ag3PO4), 16.7 (TiO2), 31.0 (Ag3PO4-10 wt% TiO2), 66.4 (Ag3PO4-25 wt% TiO2), 71.8 (Ag3PO4-50 wt% TiO2) and 79.6% (Ag3PO4-75 wt% TiO2) of the methylene blue dye was removed by adsorption. This informed the authors to conduct a detailed adsorption kinetics and equilibrium test. The photocatalytic activity of TiO2 on methylene blue was low (16.7%, k = 0.0175 min−1 within 4 min). Within the same time frame, Ag3PO4 recorded a photocatalytic efficiency of 79% with a rate constant of 0.3573 min−1. From Fig. 7, it is observed that the Ag3PO4–TiO2 composite is much more active photocatalytically than Ag3PO4 and TiO2. The photocatalytic efficiency and the rate constants were 96.3% and 0.7338 min−1, 99.1% and 0.9010 min−1, 97.7% and 0.7069 min−1 and, 94.8% and 0.3743 min−1, for Ag3PO4-10 wt% TiO2, Ag3PO4-25 wt% TiO2, Ag3PO4-50 wt% TiO2 and Ag3PO4-75 wt% TiO2, respectively. Again, the Ag3PO4-25 wt% TiO2 recorded the highest photocatalytic efficiency and rate constant.
Fig. 7. (A) Photocatalytic activities of Ag3PO4, TiO2 and Ag3PO4–TiO2 (10, 25, 50, and 75 wt% of TiO2) and (B) rate constants of Ag3PO4 (A), TiO2 (T) and Ag3PO4–TiO2 (A-wt% T) on 8 mg L−1 methylene blue dye.
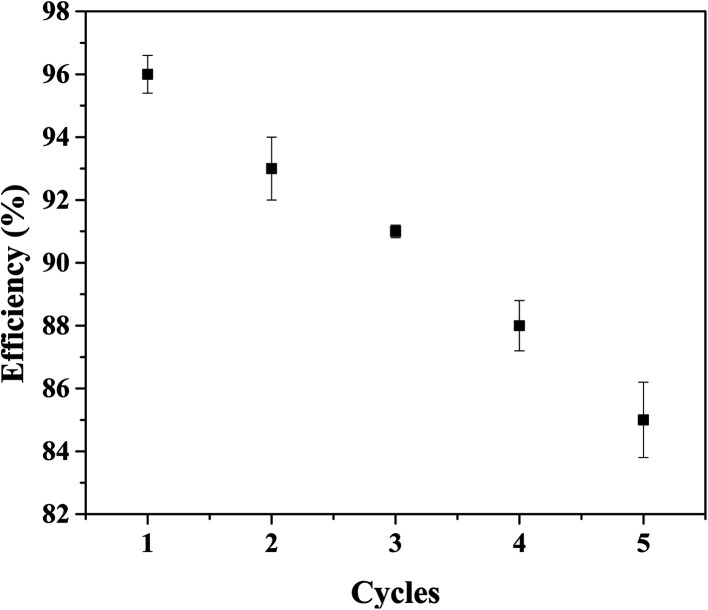
The stability of the synthesized nanocomposite was examine using Ag3PO4-50 wt% TiO2 and rhodamine B. The results for the stability/reusability of the nanocomposite is presented in Fig. 8. After five cycles of the photodegradation test, the degradation efficiency decreased from 96% to 85%. The developed Ag3PO4–TiO2 nanocomposite is therefore fairly stable under the experimental conditions used in this study.
Fig. 8. The reusability of Ag3PO4–TiO2 nanocomposite.
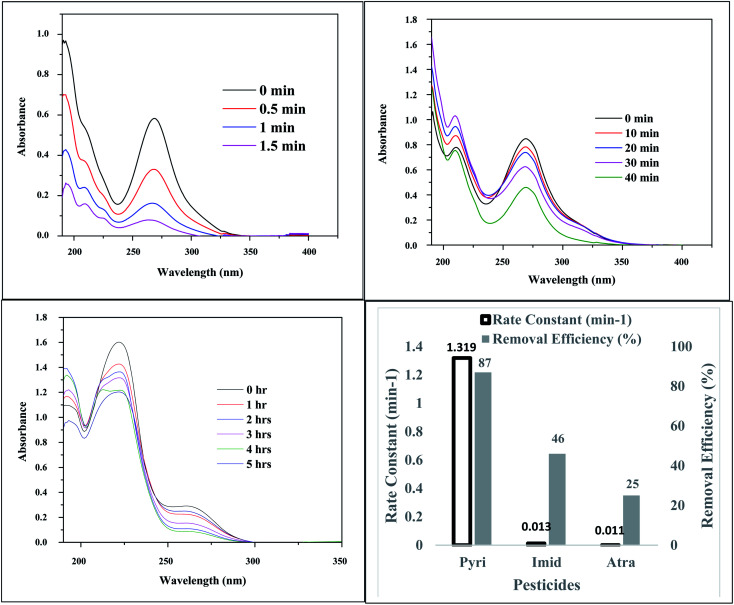
The photocatalytic activity of Ag3PO4-25 wt% TiO2 on three pesticides (pyrimethanil, atrazine and imidacloprid) was examined (Fig. 9). The Uv-vis absorption spectrum in Fig. 9 indicated a reduction in the concentration of the pesticides after photocatalysis. Though the concentration of the three pesticides are the same, the rate of degradation differed from one pesticide to the other. The pseudo first order rate constants were calculated to be 1.319, 0.013 and 0.011 min−1 for pyrimethanil, imidacloprid and atrazine, respectively. The variation in the calculated rate constants can be attributed to the different stabilities of the pesticides used for this study. The photoproducts formed from the photodegradation of pyrimethanil were identified with LC-MS.
Fig. 9. Ag3PO4-25 wt% TiO2 photodegradation of (A) 10 mg L−1 pyrimethanil, (B) 10 mg L−1 imidacloprid, (C) 10 mg L−1 atrazine; (D) calculated rate constants and removal efficiencies.
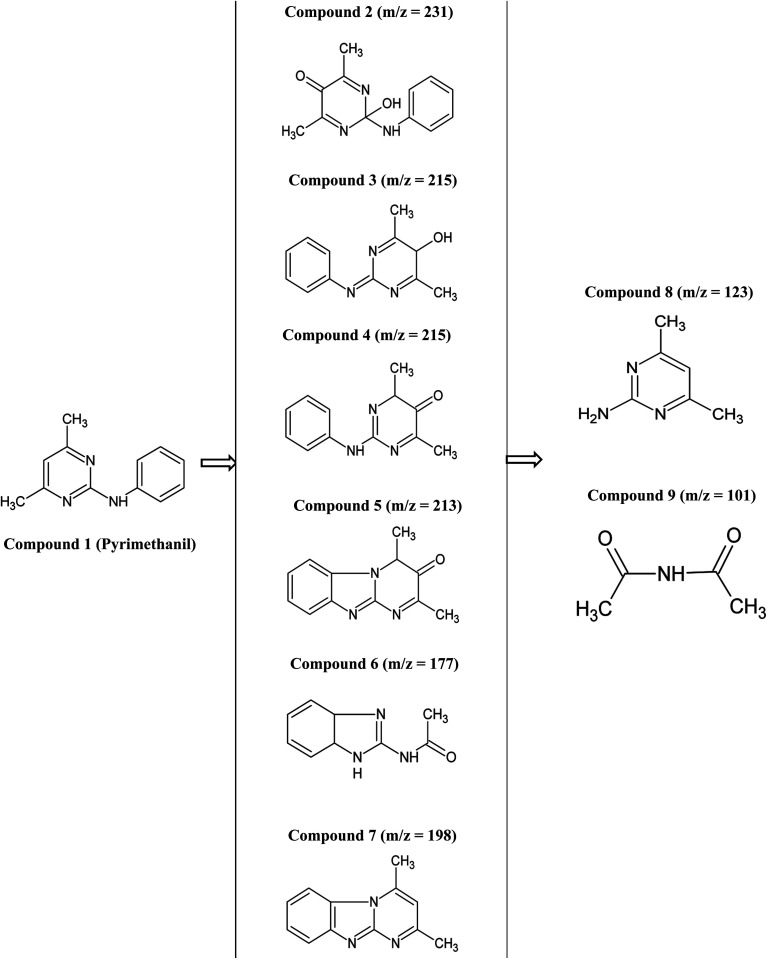
The first step in the photocatalytic degradation of pyrimethanil is the electrophilic substitution of one or two hydrogen atom in the pyrimethanil structure by hydroxyl radical.35 Electrophilic substitution will form compounds 2 (m/z = 231, tR = 1.944 min), 3 (m/z = 215, tR = 2.034), 4 (m/z 215, tR = 2.156 min), 5 (m/z = 213, tR = 1.653 min), 6 (m/z = 177, tR = 1.852 min) and 7 (m/z = 198, tR = 3.195 min). Monohydroxylation of pyrimethanil will result in the formation of compound 4 with m/z 215 (tR = 2.034 min). Compound 8 (tR = 1.354 min) was formed by the hydrolysis of pyrimethanil molecule with the loss of benzene ring. Aliphatic intermediate such as compound 9 (tR = 5.049 min) was also identified. Most of the intermediate compounds identified in this study were also identified by other researchers after photocatalytically degrading pyrimethanil.36 The structure of the compounds identified by LC-MS after the photocatalytic degradation of pyrimethanil are presented in Fig. 10.
Fig. 10. The intermediate photoproducts identified by LC-MS after photodegrading pyrimethanil.
It is obvious from the present study that the Ag3PO4–TiO2 composite has an enhanced photocatalytic activity when compared to pure Ag3PO4 and TiO2. The photocatalytic activity of TiO2 can be enhanced by coupling it with other semiconductors. Recently published work on Ag3PO4–TiO2 has suggested that the enhanced photocatalytic activity can be attributed to the unique band matching between TiO2 and Ag3PO4, inhibition of the electron hole recombination by TiO2, and the inter semiconductor hole transfer between the valence bands of TiO2 and Ag3PO4.29a,33,37
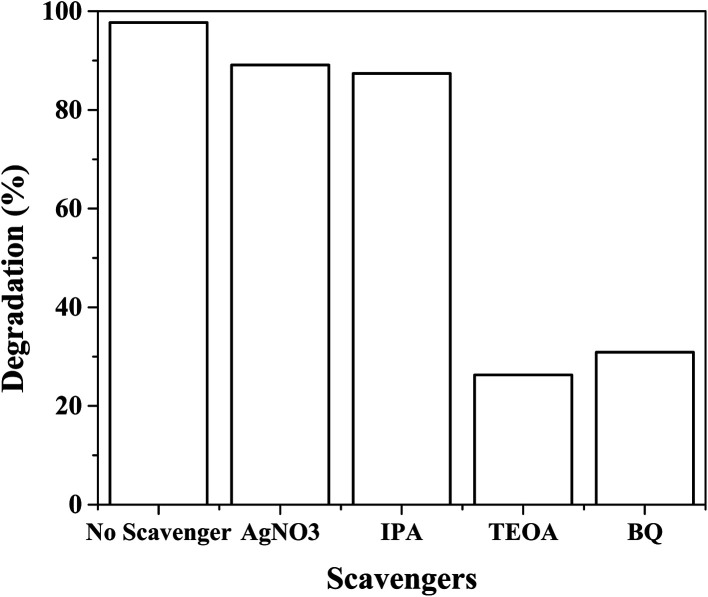
Hydroxyl radicals, photogenerated electrons and holes, and superoxide radicals are the reactive species that are responsible for the photodegradation of organic pollutants. Benzoquinone (BQ), AgNO3, isopropyl alcohol (IPA) and triethanolamine (TEOA) are effective scavengers for superoxide radicals (˙O2−), photogenerated electrons (e−), hydroxyl radicals (˙OH) and photogenerated holes (h+), respectively.27,30 These scavengers were therefore used to examine which of the reactive species are responsible for the degradation of rhodamine blue dye. Using Ag3PO4-25 wt% TiO2, and rhodamine B solution, upon the addition of AgNO3, IPA, TEOA and BQ, the degradation efficiency recorded reduced from 98% to 89.1%, 87.4%, 26.3% and 30.9%, respectively (Fig. 11). Therefore, photogenerated holes and superoxide radicals are the main reactive species, responsible for the photodegradation of rhodamine B dye by Ag3PO4-25 wt% TiO2. This results is in agreement with the research findings of Zhang Yang et al.27
Fig. 11. Effect of scavengers on the photodegradation of rhodamine B by Ag3PO4-25 wt% TiO2.
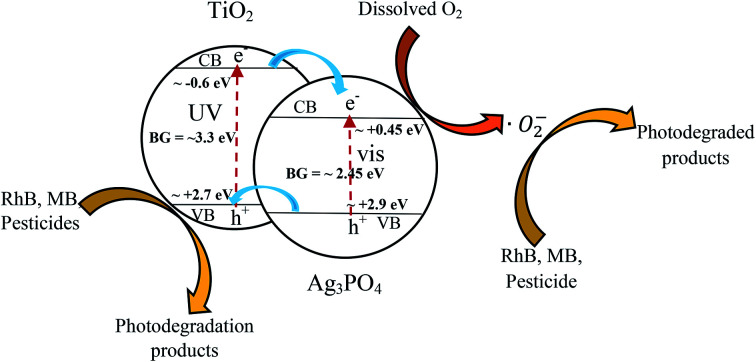
A possible explanation to the trend observed in this study is schematically presented in Fig. 12.
Fig. 12. Diagram for electron (e−) and Hole (h+) separation, generation of reactive oxygen species and degradation of rhodamine B (RhB), methylene blue (MB) and pesticides [BG = Band Gap].
Ag3PO4 and TiO2 can be excited by visible and UV light, resulting in the generation of photo excited electrons and holes. Under UV-visible light irradiation, the electrons in the valence band of Ag3PO4 are excited into the conduction band resulting in the generation of holes in the valence band of Ag3PO4. Since the valence band maxima position of TiO2 (+2.7 eV vs. NHE) is relatively higher than that of Ag3PO4 (+2.9 eV vs. NHE),33,37 the holes in the valence band of Ag3PO4 will then be transferred into the valence band of TiO2 while the photogenerated electrons will move in the conduction band of the Ag3PO4. This charge transfer is possible if and only if a heterojunction is created between the TiO2 and the Ag3PO4. It can be seen from the SEM images in Fig. 3 that the TiO2 covered the surface of the Ag3PO4 allowing this heterojunction to be created. The photogenerated electrons and holes are therefore separated reducing the electron–hole recombination and subsequently enhancing the photocatalytic activity. The holes generated in the valence band of the TiO2 can be used to oxidize pollutants such as dye and pesticides resulting in their degradation. In addition, the photogenerated electrons in the conduction band will react with dissolved oxygen to generate superoxide radicals. These superoxide radicals will attach pollutants and degrade them. The reactive species responsible for the degradation of organic dyes and pesticides are photogenerated holes (h+) and superoxide radicals (˙O2−).
The photocatalytic activity of Ag3PO4–TiO2 photocatalyst from this work is compared with those reported in literature. It can be seen that, the photodegradation activity of the Ag3PO4–TiO2 synthesized from this work is high (Table 1).
Comparison of the photocatalytic activity of Ag3PO4–TiO2 towards the degradation of organic pollutants.
| Pollutant | Photocatalyst amount | Light intensity | Irradiation time | Degradation % | Ref. |
|---|---|---|---|---|---|
| Rhodamine B (200 mL, 6 mg L−1) | 100 mg | 300 W tungsten halogen lamp | 6 min | 98% | This work |
| Methylene blue (200 mL, 8 mg L−1) | 100 mg | 300 W tungsten halogen lamp | 4 min | 99.1% | |
| Rhodamine B (50 mL, 10 mg L−1) | 50 mg | 500 W Xe lamp | 50 min | 99.11% | 38 |
| Methyl orange (40 mL, 10 mg L−1) | 40 mg | 300 W Xe lamp | 60 min | 95.5% | 39 |
| Methylene blue (130 mL, 20 mg L−1) | 65 mg | 150 W Xe lamp | 28 min | ∼100% | 29b |
| Rhodamine B (100 mL, 10 mg L−1) | 40 mg | 300 W Xe lamp | 10 min | ∼100% | 40 |
| Bisphenol A (120 mL, 220 ug/L) | 30 mg | 100 W Xe lamp | 10 min | ∼100% | 33 |
| Methylene blue (80 mL, 10 mM) | 0.03 M | 500 W xenon lamp | 160 min | 97.1% | 41 |
| 16 W high pressure lamp | 70 min | 98.1% | |||
| Rhodamine B (100 mL, 0.02 mM) | 50 mg | 300 W Xe lamp | 12 min | ∼100% | 29a |
| Rhodamine B (100 mL, 10 mg L−1) | 50 mg | 500 W Xe lamp | 12 min | ∼100% | 30 |
| Methylene blue (75 mL, 10 mg L−1) | 40 mg | 500 W high pressure lamp | 12 min | ∼95% | 42 |
| Methyl orange (20 mL, 20 mg L−1) | 20 mg | 300 W Hg lamp | 30 min | ∼100% | 43 |
3.3. Adsorption equilibrium and kinetic studies
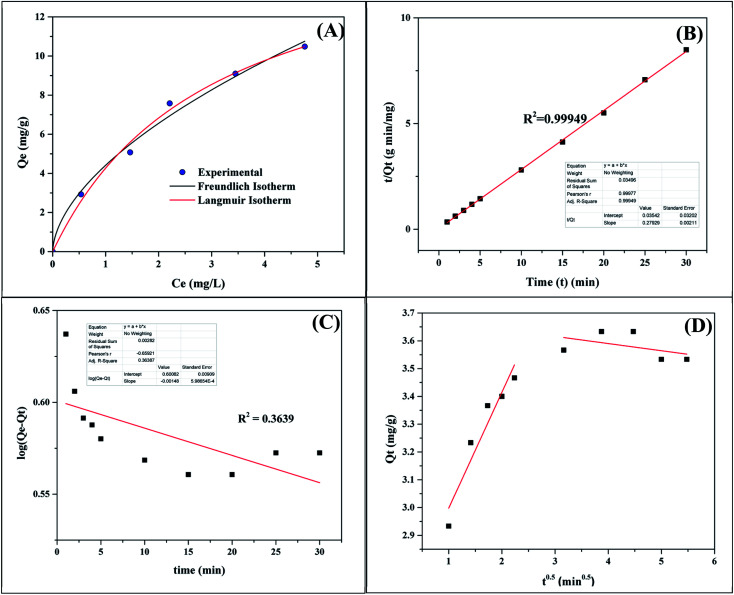
The adsorption equilibrium and kinetic studies were conducted using 200 mL of 5 mg L−1 methylene blue solution and 100 mg of Ag3PO4-50 wt% TiO2. Fig. 13 and Table 2 contain the plots and data obtained from the adsorption studies. From Fig. 13A the amount of dye adsorbed at equilibrium (Qe) increased with increasing equilibrium concentration of the dye (Ce). To obtain a better understanding of the adsorption process, the experimental data was fitted with the Langmuir and Freundlich equilibrium isotherms. The Langmuir isotherm is given as;
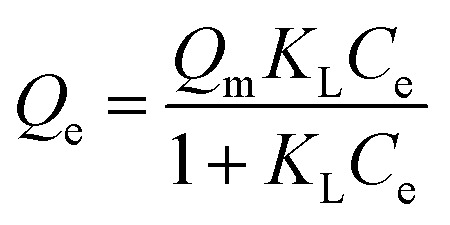 |
1 |
In this equation, Ce (mg L−1) is the equilibrium concentration of the dye, Qe (mg g−1) is the amount of dye adsorbed at equilibrium, Qm (mg g−1) is the maximum adsorption capacity corresponding to a monolayer coverage and KL (L mg−1) is the Langmuir constant. The plot of Qevs. Ce is presented in Fig. 13A. Qm and KL can be estimated from the slope and intercept of linearized Langmuir isotherm expression. The estimated Qm and KL are presented in Table 2. To examine the favorability or otherwise of the adsorption process, an equilibrium parameter RL was calculated from the equation below and the values presented in Table 2.
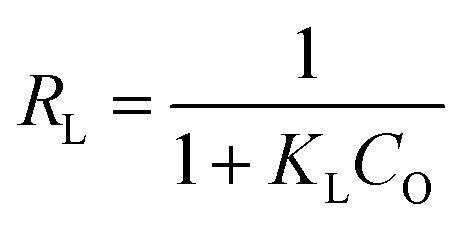 |
2 |
KL (L mg−1) is the Langmuir constant and Co (mg L−1) is the initial dye concentration. The RL value indicates adsorption process is irreversible when RL is 0; favorable when RL is between 0 and 1; linear when RL is 1 and unfavorable when RL is greater than 1. The maximum monolayer adsorption capacity (Qm) and the equilibrium parameter (RL) of the Ag3PO4-50 wt% TiO2 were calculated to be 17.112 mg g−1 and 0.231–0.600, respectively. These values imply that the surface of the Ag3PO4-50 wt% TiO2 is homogenous and was covered with a monolayer methylene blue dye. In addition, the adsorption of methylene blue by Ag3PO4-50 wt% TiO2 was favorable.
Fig. 13. (A) Langmuir and Freundlich adsorption isotherms, (B) pseudo second order (C) pseudo first order kinetics and (D) interparticle diffusion model for the adsorption of methylene blue by Ag3PO4-50 wt% TiO2.
Calculated adsorption isotherm and equilibrium kinetics parameters for the adsorption of methylene blue by Ag3PO4-50 wt% TiO2.
| Adsorption kinetic models | Adsorption kinetic parameters |
|---|---|
| Langmuir | Q m = 17.112 (mg g−1) |
| K L = 0.3324 (L mg−1) | |
| R 2 = 0.9920 | |
| R L = 0.231–0.6 (L mg−1) | |
| Freundlich | K F = 4.4181 (mg g−1 (mg L−1)1/n) |
| n = 1.7531 | |
| R 2 = 0.9888 | |
| Pseudo first order | Q e = 3.9886 (mg g−1) |
| K 1 = 0.00341 (min−1) | |
| R 2 = 0.3639 | |
| Pseudo second order | Q e = 3.5804 (mg g−1) |
| K 2 = 2.2036 (g mg−1 min−1) | |
| R 2 = 0.9995 | |
| Interparticle diffusion model | K P = 2.5812 (mg g−1 min−1 ½) |
| R 2 = 0.9189 |
In addition to the Langmuir equilibrium model, the adsorption data was fitted with the Freundlich equation. The Freundlich equation is presented below;
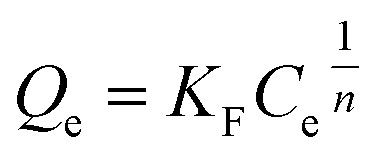 |
3 |
Qe (mg g−1) is the amount of dye adsorbed on Ag3PO4-50 wt% TiO2 at equilibrium, Ce is the equilibrium concentration of the dye in solution, KF (mg g−1(mg L−1)1/n) and 1/n are the Freundlich constants related to the adsorption capacity and the adsorption intensity, respectively. Higher values of KF indicate higher affinity for adsorption and the values of 1/n lie between 0.1 < 1/n < 1, indicating favorable adsorption.44 The Freundlich constants were calculated from the linearized Freundlich isotherm expression and presented in Table 2. The value of 1/n is less than 1 indicating favorable adsorption process. The KF and a correlation coefficient were estimated as 4.4181 mg g−1 (mg L−1)1/n and 0.9888, respectively. Since the correlation coefficient for both the Langmuir and Freundlich model were greater than 0.95, the adsorption process may have followed both models. It is however likely the adsorption process followed the Langmuir isotherm model since it had a relatively higher correlation coefficient when compared to Freundlich isotherm.
The mechanism and rate controlling step of the entire adsorption process was examined using the following adsorption kinetic models; pseudo-first order, pseudo-second order and intra-particle diffusion models. The pseudo-first order equation is presented below;
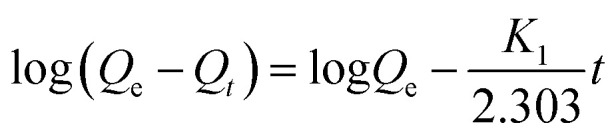 |
4 |
Qe and Qt (mg g−1) are the amount of the dye adsorbed at equilibrium and at time t (min), respectively. K1 (min−1) is the pseudo-first order rate constant. The parameters K1 and Qe were estimated from Fig. 13C and presented in Table 2. The correlation coefficient obtained is low (R2 = 0.3639). This suggests that the pseudo-first order kinetic model is not suitable to describe the adsorption process.
The adsorption kinetics was also investigated using pseudo-second order kinetic model presented below;
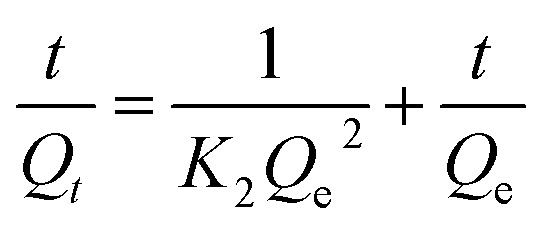 |
5 |
K2 (g mg−1 min−1) is the pseudo-second order rate constant. Qe and Qt (mg g−1) are the amount of dye adsorbed at equilibrium and at time t (min), respectively. Qe and K2 were calculated from Fig. 13B. The high correlation coefficient of 0.9995 suggests that the adsorption process follows a pseudo-second order adsorption kinetics process.
The Langmuir adsorption isotherm confirm the monolayer adsorption of methylene blue onto the surface of the Ag3PO4-50 wt% TiO2. The dye molecules were probably transported from the dye solution to the Ag3PO4-50 wt% TiO2. The possibility of intra-particle diffusion was therefore examined using the intra-particle diffusion model45 presented below;
| Qt = Kpt1/2 + C | 6 |
Qt (mg g−1) is the amount of methylene blue dye adsorbed at time t (min), C is the intercept and Kp (mg g−1 min−1) is the intra-particle diffusion rate constant. Kp was estimated from Fig. 13D and presented in Table 2. The plot in Fig. 13D shows a double straight line. The initial stage represents the transportation of methylene blue to the external surface of the Ag3PO4-50 wt% TiO2 through film diffusion within a very short time. The first linear part can be due to the entry of methylene blue molecules into the Ag3PO4-50 wt% TiO2 by intra-particle diffusion.46 The final equilibrium stage is represented by the second linear portion of Fig. 13D.
The results presented in this article revealed that TiO2–Ag3PO4 has the potential of removing water pollutants through adsorption and photocatalysis. TiO2–Ag3PO4 therefore has a potential application in the development of water treatment technologies.
4. Conclusion
In this study, TiO2 semiconductor was coupled with Ag3PO4. SEM images of the as-prepared TiO2–Ag3PO4 showed a covering of the Ag3PO4 surface by TiO2 creating a heterojunction between coupled semiconductors. DRS analysis and optical band gap estimation revealed two absorption thresholds corresponding to TiO2 and Ag3PO4. TiO2–Ag3PO4 showed strong photocatalytic activity for rhodamine B, methylene blue, pyrimethanil, imidacloprid and atrazine. LC-MS analysis of the photodegraded pyrimethanil resulted in the identification of hydroxylated and aliphatic derivatives of pyrimethanil. The photocatalytic activity was dependent on the weight percent of the TiO2 used in the synthesis with 25 wt% TiO2 recording the highest photocatalytic removal efficiency and rate constant. The synergy between the TiO2 and Ag3PO4 semiconductors was attributed to the good band gap matching that resulted in the efficient separation of charges which in turn led to a suppression of the electron–hole recombination. The TiO2–Ag3PO4 showed strong adsorption towards methylene blue dye and the adsorption equilibrium isotherm and kinetics followed the Freundlich and Langmuir isotherms, and pseudo second order rate kinetics, respectively. TiO2–Ag3PO4 therefore removes the pollutants by both photocatalytic and adsorption process. The results from this study showed the potential application of TiO2–Ag3PO4 in water treatment technologies.
Conflicts of interest
There is no conflict to declare.
Supplementary Material
Acknowledgments
This work was financially supported through Commonwealth Academic Fellowship funded by the UK Government.
References
- (a) Konstantinou I. K. Albanis T. A. Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways. Appl. Catal., B. 2003;42(4):319–335. doi: 10.1016/S0926-3373(02)00266-7. [DOI] [Google Scholar]; (b) Daneshvar N. Salari D. Khataee A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol., A. 2004;162(2–3):317–322. doi: 10.1016/S1010-6030(03)00378-2. [DOI] [Google Scholar]; (c) Bailey S. E. Olin T. J. Bricka R. M. Adrian D. D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999;33(11):2469–2479. doi: 10.1016/S0043-1354(98)00475-8. [DOI] [Google Scholar]; (d) Kim S. D. Cho J. Kim I. S. Vanderford B. J. Snyder S. A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007;41(5):1013–1021. doi: 10.1016/j.watres.2006.06.034. [DOI] [PubMed] [Google Scholar]; (e) Nyankson E. Kumar R. Removal of water-soluble dyes and pharmaceutical wastes by combining the photocatalytic properties of Ag3PO4 with the adsorption properties of halloysite nanotubes. Materials Today Advances. 2019;4:100025. doi: 10.1016/j.mtadv.2019.100025. [DOI] [Google Scholar]
- Konstantinou I. K. Albanis T. A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review. Appl. Catal., B. 2004;49(1):1–14. doi: 10.1016/j.apcatb.2003.11.010. [DOI] [Google Scholar]
- Ràfols C. Barceló D. Determination of mono-and disulphonated azo dyes by liquid chromatography–atmospheric pressure ionization mass spectrometry. J. Chromatogr. A. 1997;777(1):177–192. doi: 10.1016/S0021-9673(97)00429-9. [DOI] [Google Scholar]
- Lhomme L. Brosillon S. Wolbert D. Photocatalytic degradation of pesticides in pure water and a commercial agricultural solution on TiO2 coated media. Chemosphere. 2008;70(3):381–386. doi: 10.1016/j.chemosphere.2007.07.004. [DOI] [PubMed] [Google Scholar]
- (a) Barbash J. E. Thelin G. P. Kolpin D. W. Gilliom R. J. Major herbicides in ground water. J. Environ. Qual. 2001;30(3):831–845. doi: 10.2134/jeq2001.303831x. [DOI] [PubMed] [Google Scholar]; (b) Fenoll J. Flores P. Hellín P. Martínez C. M. Navarro S. Photodegradation of eight miscellaneous pesticides in drinking water after treatment with semiconductor materials under sunlight at pilot plant scale. Chem. Eng. J. 2012;204:54–64. doi: 10.1016/j.cej.2012.07.077. [DOI] [Google Scholar]
- (a) Shen Y. Xu Q. Wei R. Ma J. Wang Y. Mechanism and dynamic study of reactive red X-3B dye degradation by ultrasonic-assisted ozone oxidation process. Ultrason. Sonochem. 2017;38:681–692. doi: 10.1016/j.ultsonch.2016.08.006. [DOI] [PubMed] [Google Scholar]; (b) Tawk A. Deborde M. Labanowski J. Thibaudeau S. Gallard H. Transformation of the B-triketone pesticides tembotrione and sulcotrione by reactions with ozone: Kinetic study, transformation products, toxicity and biodegradability. Ozone: Sci. Eng. 2017;39(1):3–13. doi: 10.1080/01919512.2016.1257933. [DOI] [Google Scholar]; (c) Derylo-Marczewska A. Blachnio M. Marczewski A. W. Swiatkowski A. Buczek B. Adsorption of chlorophenoxy pesticides on activated carbon with gradually removed external particle layers. Chem. Eng. J. 2017;308:408–418. doi: 10.1016/j.cej.2016.09.082. [DOI] [Google Scholar]; (d) Sohrabi M. R. Khavaran A. Shariati S. Shariati S. Removal of Carmoisine edible dye by Fenton and photo Fenton processes using Taguchi orthogonal array design. Arabian J. Chem. 2017;10:S3523–S3531. doi: 10.1016/j.arabjc.2014.02.019. [DOI] [Google Scholar]
- Forgacs E. Cserhati T. Oros G. Removal of synthetic dyes from wastewaters: a review. Environ. Int. 2004;30(7):953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- (a) Cruz M. Gomez C. Duran-Valle C. J. Pastrana-Martínez L. M. Faria J. L. Silva A. M. Faraldos M. Bahamonde A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017;416:1013–1021. doi: 10.1016/j.apsusc.2015.09.268. [DOI] [Google Scholar]; (b) Yang S.-F. Niu C.-G. Huang D.-W. Zhang H. Liang C. Zeng G.-M. SrTiO 3 nanocubes decorated with Ag/AgCl nanoparticles as photocatalysts with enhanced visible-light photocatalytic activity towards the degradation of dyes, phenol and bisphenol A. Environ. Sci.: Nano. 2017;4(3):585–595. doi: 10.1039/C6EN00597G. [DOI] [Google Scholar]; (c) Agbe H. Nyankson E. Raza N. Dodoo-Arhin D. Chauhan A. Osei G. Kumar V. Kim K.-H. Recent advances in photoinduced catalysis for water splitting and environmental applications. J. Ind. Eng. Chem. 2019;72:31–49. doi: 10.1016/j.jiec.2019.01.004. [DOI] [Google Scholar]; (d) Nyankson E. Agyei-Tuffour B. Adjasoo J. Ebenezer A. Dodoo-Arhin D. Yaya A. Mensah B. Efavi J. K. Synthesis and application of Fe-doped TiO2-halloysite nanotubes composite and their potential application in water treatment. Adv. Mater. Sci. Eng. 2019:2019. [Google Scholar]
- Agyei-Tuffour B. Gbogbo S. Dodoo-Arhin D. Damoah L. N. Efavi J. K. Yaya A. Nyankson E. Photocatalytic degradation of fractionated crude oil: potential application in oil spill remediation. Cogent Engineering. 2020;7(1):1744944. doi: 10.1080/23311916.2020.1744944. [DOI] [Google Scholar]
- Dodoo-Arhin D. Bowen-Dodoo E. Agyei-Tuffour B. Nyankson E. Obayemi J. D. Salifu A. A. Yaya A. Agbe H. Soboyejo W. O. Modified nanostructured titania photocatalysts for aquatic disinfection applications. Mater. Today: Proc. 2020;38:1183–1190. [Google Scholar]
- Oller I. Gernjak W. Maldonado M. Pérez-Estrada L. Sánchez-Pérez J. Malato S. Solar photocatalytic degradation of some hazardous water-soluble pesticides at pilot-plant scale. J. Hazard. Mater. 2006;138(3):507–517. doi: 10.1016/j.jhazmat.2006.05.075. [DOI] [PubMed] [Google Scholar]
- (a) Kumar A. Kumar K. Krishnan V. Sunlight driven methanol oxidation by anisotropic plasmonic Au nanostructures supported on amorphous titania: Influence of morphology on photocatalytic activity. Mater. Lett. 2019;245:45–48. doi: 10.1016/j.matlet.2019.02.091. [DOI] [Google Scholar]; (b) Kumar A. Sharma V. Kumar S. Kumar A. Krishnan V. Towards utilization of full solar light spectrum using green plasmonic Au–TiOx photocatalyst at ambient conditions. Surf. Interfaces. 2018;11:98–106. doi: 10.1016/j.surfin.2018.03.005. [DOI] [Google Scholar]
- Lu Z. Zeng L. Song W. Qin Z. Zeng D. Xie C. In situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal., B. 2017;202:489–499. doi: 10.1016/j.apcatb.2016.09.052. [DOI] [Google Scholar]
- Ismael M. Elhaddad E. Taffa D. H. Wark M. Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst. Catalysts. 2017;7(11):326. doi: 10.3390/catal7110326. [DOI] [Google Scholar]
- Bora T. Sathe P. Laxman K. Dobretsov S. Dutta J. Defect engineered visible light active ZnO nanorods for photocatalytic treatment of water. Catal. Today. 2017;284:11–18. doi: 10.1016/j.cattod.2016.09.014. [DOI] [Google Scholar]
- Harish S. Archana J. Sabarinathan M. Navaneethan M. Nisha K. Ponnusamy S. Muthamizhchelvan C. Ikeda H. Aswal D. Hayakawa Y. Controlled structural and compositional characteristic of visible light active ZnO/CuO photocatalyst for the degradation of organic pollutant. Appl. Surf. Sci. 2017;418:103–112. doi: 10.1016/j.apsusc.2016.12.082. [DOI] [Google Scholar]
- Li Y.-H. Li J.-Y. Xu Y.-J. Bimetallic Nanoparticles as Cocatalysts for Versatile Photoredox Catalysis. Surf. Interfaces. 2020:100047. [Google Scholar]
- (a) Zhang F. Li Y.-H. Li J.-Y. Tang Z.-R. Xu Y.-J. 3D graphene-based gel photocatalysts for environmental pollutants degradation. Environ. Pollut. 2019;253:365–376. doi: 10.1016/j.envpol.2019.06.089. [DOI] [PubMed] [Google Scholar]; (b) Lu K.-Q. Xin X. Zhang N. Tang Z.-R. Xu Y.-J. Photoredox catalysis over graphene aerogel-supported composites. J. Mater. Chem. A. 2018;6(11):4590–4604. doi: 10.1039/C8TA00728D. [DOI] [Google Scholar]
- (a) Yi Z. Ye J. Kikugawa N. Kako T. Ouyang S. Stuart-Williams H. Yang H. Cao J. Luo W. Li Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010;9(7):559. doi: 10.1038/nmat2780. [DOI] [PubMed] [Google Scholar]; (b) Nyankson E. Amedalor R. Chandrabose G. Coto M. Krishnamurthy S. Kumar R. V. Microwave-and Formaldehyde-Assisted Synthesis of Ag–Ag3PO4 with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B Dye and Crude Oil Fractions. ACS Omega. 2020;5(23):13641–13655. doi: 10.1021/acsomega.0c00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y. Ouyang S. Cao J. Ye J. Facile synthesis of rhombic dodecahedral AgX/Ag 3 PO 4 (X= Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011;13(21):10071–10075. doi: 10.1039/C1CP20488B. [DOI] [PubMed] [Google Scholar]
- Chen Z. Wang W. Zhang Z. Fang X. High-efficiency visible-light-driven Ag3PO4/AgI photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic activity. J. Phys. Chem. C. 2013;117(38):19346–19352. doi: 10.1021/jp406508y. [DOI] [Google Scholar]
- Katsumata H. Sakai T. Suzuki T. Kaneco S. Highly efficient photocatalytic activity of g-C3N4/Ag3PO4 hybrid photocatalysts through Z-scheme photocatalytic mechanism under visible light. Ind. Eng. Chem. Res. 2014;53(19):8018–8025. doi: 10.1021/ie5012036. [DOI] [Google Scholar]
- Zhang H. Huang H. Ming H. Li H. Zhang L. Liu Y. Kang Z. Carbon quantum dots/Ag 3 PO 4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light. J. Mater. Chem. 2012;22(21):10501–10506. doi: 10.1039/C2JM30703K. [DOI] [Google Scholar]
- Guan X. Guo L. Cocatalytic effect of SrTiO3 on Ag3PO4 toward enhanced photocatalytic water oxidation. ACS Catal. 2014;4(9):3020–3026. doi: 10.1021/cs5005079. [DOI] [Google Scholar]
- Dodoo-Arhin D. Buabeng F. P. Mwabora J. M. Amaniampong P. N. Agbe H. Nyankson E. Obada D. O. Asiedu N. Y. The effect of titanium dioxide synthesis technique and its photocatalytic degradation of organic dye pollutants. Heliyon. 2018;4(7):e00681. doi: 10.1016/j.heliyon.2018.e00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Zhang L. Zhang H. Huang H. Liu Y. Kang Z. Ag 3 PO 4/SnO 2 semiconductor nanocomposites with enhanced photocatalytic activity and stability. New J. Chem. 2012;36(8):1541–1544. doi: 10.1039/C2NJ40206H. [DOI] [Google Scholar]; (b) Jia Y. Ma Y. Zhu L. Dong J. Lin Y. Efficient visible-light-responsive photocatalyst: hybrid TiO2-Ag3PO4 nanorods. Chem. Phys. 2019;521:1–4. doi: 10.1016/j.chemphys.2019.01.015. [DOI] [Google Scholar]
- Zhang Y. Duoerkun G. Shi Z. Cao W. Liu T. Liu J. Zhang L. Li M. Chen Z. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 2020;571:213–221. doi: 10.1016/j.jcis.2020.03.049. [DOI] [PubMed] [Google Scholar]
- Al Kausor M. Chakrabortty D. Facile fabrication of N-TiO2/Ag3PO4@ GO nanocomposite toward photodegradation of organic dye under visible light. Inorg. Chem. Commun. 2020;116:107907. doi: 10.1016/j.inoche.2020.107907. [DOI] [Google Scholar]
- (a) Zhao F.-M. Pan L. Wang S. Deng Q. Zou J.-J. Wang L. Zhang X. Ag3PO4/TiO2 composite for efficient photodegradation of organic pollutants under visible light. Appl. Surf. Sci. 2014;317:833–838. doi: 10.1016/j.apsusc.2014.09.022. [DOI] [Google Scholar]; (b) Li Y. Yu L. Li N. Yan W. Li X. Heterostructures of Ag3PO4/TiO2 mesoporous spheres with highly efficient visible light photocatalytic activity. J. Colloid Interface Sci. 2015;450:246–253. doi: 10.1016/j.jcis.2015.03.016. [DOI] [PubMed] [Google Scholar]; (c) Hamrouni A. Azzouzi H. Rayes A. Palmisano L. Ceccato R. Parrino F. Enhanced solar light photocatalytic activity of ag doped TiO2–Ag3PO4 composites. Nanomaterials. 2020;10(4):795. doi: 10.3390/nano10040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Duan W. Jiang H. Luo S. Luo Y. Mesoporous TiO2@ Ag3PO4 photocatalyst with high adsorbility and enhanced photocatalytic activity under visible light. Mater. Res. Bull. 2015;70:129–136. doi: 10.1016/j.materresbull.2015.04.029. [DOI] [Google Scholar]
- Li C. Zhang P. Lv R. Lu J. Wang T. Wang S. Wang H. Gong J. Selective deposition of Ag3PO4 on monoclinic BiVO4 (040) for highly efficient photocatalysis. Small. 2013;9(23):3951–3956. doi: 10.1002/smll.201301276. [DOI] [PubMed] [Google Scholar]
- Zanella R. Giorgio S. Henry C. R. Louis C. Alternative methods for the preparation of gold nanoparticles supported on TiO2. J. Phys. Chem. B. 2002;106(31):7634–7642. doi: 10.1021/jp0144810. [DOI] [Google Scholar]
- Taheri M. E. Petala A. Frontistis Z. Mantzavinos D. Kondarides D. I. Fast photocatalytic degradation of bisphenol A by Ag3PO4/TiO2 composites under solar radiation. Catal. Today. 2017;280:99–107. doi: 10.1016/j.cattod.2016.05.047. [DOI] [Google Scholar]
- López R. Gómez R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: a comparative study. J. Sol-Gel Sci. Technol. 2012;61(1):1–7. doi: 10.1007/s10971-011-2582-9. [DOI] [Google Scholar]
- (a) Sirtori C. Zapata A. Malato S. Agüera A. Formation of chlorinated by-products during photo-Fenton degradation of pyrimethanil under saline conditions. Influence on toxicity and biodegradability. J. Hazard. Mater. 2012;217–218:217–223. doi: 10.1016/j.jhazmat.2012.03.017. [DOI] [PubMed] [Google Scholar]; (b) Kinani A. Rifai A. Bourcier S. Jaber F. Bouchonnet S. Structural characterization of photoproducts of pyrimethanil. J. Mass Spectrom. 2013;48(8):983–987. doi: 10.1002/jms.3244. [DOI] [PubMed] [Google Scholar]
- Agueera A. Almansa E. Tejedor A. Fernandez-Alba A. R. Malato S. Maldonado M. I. Photocatalytic pilot scale degradation study of pyrimethanil and of its main degradation products in waters by means of solid-phase extraction followed by gas and liquid chromatography with mass spectrometry detection. Environ. Sci. Technol. 2000;34(8):1563–1571. doi: 10.1021/es990112u. [DOI] [Google Scholar]
- Rawal S. B. Do Sung S. Lee W. I. Novel Ag3PO4/TiO2 composites for efficient decomposition of gaseous 2-propanol under visible-light irradiation. Catal. Commun. 2012;17:131–135. doi: 10.1016/j.catcom.2011.10.034. [DOI] [Google Scholar]
- Wang P. Li Y. Liu Z. Chen J. Wu Y. Guo M. Na P. In-situ deposition of Ag3PO4 on TiO2 nanosheets dominated by (001) facets for enhanced photocatalytic activities and recyclability. Ceram. Int. 2017;43(15):11588–11595. doi: 10.1016/j.ceramint.2017.05.178. [DOI] [Google Scholar]
- Liu H. Li D. Yang X. Li H. Fabrication and characterization of Ag3PO4/TiO2 heterostructure with improved visible-light photocatalytic activity for the degradation of methyl orange and sterilization of E. coli. Mater. Technol. 2019;34(4):192–203. doi: 10.1080/10667857.2018.1545391. [DOI] [Google Scholar]
- Xie J. Yang Y. He H. Cheng D. Mao M. Jiang Q. Song L. Xiong J. Facile synthesis of hierarchical Ag3PO4/TiO2 nanofiber heterostructures with highly enhanced visible light photocatalytic properties. Appl. Surf. Sci. 2015;355:921–929. doi: 10.1016/j.apsusc.2015.07.175. [DOI] [Google Scholar]
- Tang C. Liu E. Fan J. Hu X. Kang L. Wan J. Heterostructured Ag3PO4/TiO2 nano-sheet film with high efficiency for photodegradation of methylene blue. Ceram. Int. 2014;40(10):15447–15453. doi: 10.1016/j.ceramint.2014.06.116. [DOI] [Google Scholar]
- Ma X. Li H. Wang Y. Li H. Liu B. Yin S. Sato T. Substantial change in phenomenon of “self-corrosion” on Ag3PO4/TiO2 compound photocatalyst. Appl. Catal., B. 2014;158:314–320. [Google Scholar]
- Liu R. Hu P. Chen S. Photocatalytic activity of Ag3PO4 nanoparticle/TiO2 nanobelt heterostructures. Appl. Surf. Sci. 2012;258(24):9805–9809. doi: 10.1016/j.apsusc.2012.06.033. [DOI] [Google Scholar]
- (a) Gupta V. K. Jain C. Ali I. Chandra S. Agarwal S. Removal of lindane and malathion from wastewater using bagasse fly ash—a sugar industry waste. Water Res. 2002;36(10):2483–2490. doi: 10.1016/S0043-1354(01)00474-2. [DOI] [PubMed] [Google Scholar]; (b) Özcan A. S. Erdem B. Özcan A. Adsorption of Acid Blue 193 from aqueous solutions onto Na–bentonite and DTMA–bentonite. J. Colloid Interface Sci. 2004;280(1):44–54. doi: 10.1016/j.jcis.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Doğan M. Alkan M. Türkyilmaz A. Özdemir Y. Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. J. Hazard. Mater. 2004;109(1–3):141–148. doi: 10.1016/j.jhazmat.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Luo P. Zhao Y. Zhang B. Liu J. Yang Y. Liu J. Study on the adsorption of Neutral Red from aqueous solution onto halloysite nanotubes. Water Res. 2010;44(5):1489–1497. doi: 10.1016/j.watres.2009.10.042. [DOI] [PubMed] [Google Scholar]