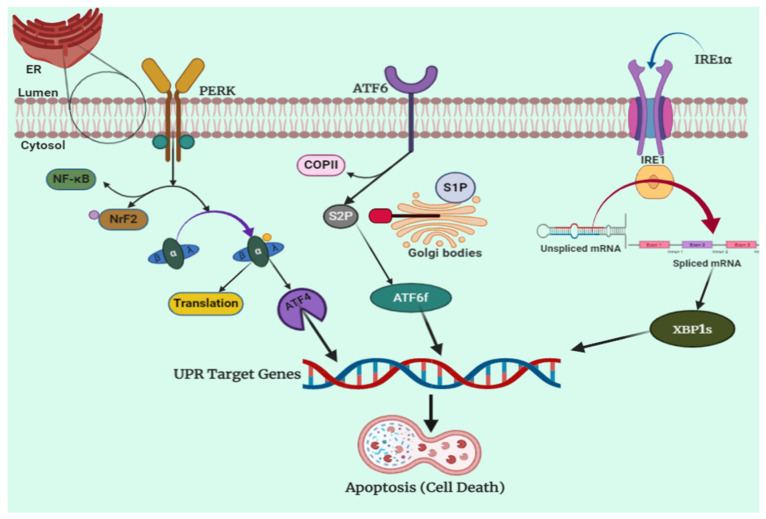
Figure 2.
Endoplasmic reticulum stress pathway. The ER stress-associated unfolding protein accumulation stimulates UPR through sensor proteins such asIRE1, ATF-6, and PERK. The ER membrane contains three sensors that detect ER stress: PERK, ATF6, and IRE1. Grp78 dissociates from each of the unfolded proteins that accumulate in the ER lumen, prompting their stimulation. When PERK dimerizes and becomes autophosphorylated, its cytosolic kinase domain is activated, phosphorylating eIF2. Cap-dependent translation of ATF4 is enabled as a result of this suppression of overall protein synthesis. Grp78 dissociation induces ATF6 translocation to the Golgi, where it is translated by site 1 and site 2 proteases (S1P and S2P) into a functional transcription factor that activates XBP1 mRNA transcription. Grp78 dissociation from IRE1 causes IRE1 dimerization and autophosphorylation. When IRE1 is activated, its endoribonuclease activity causes un-spliced XBP1 mRNA to be processed, while its kinase site recruits TRAF2 and ASK1, causing JNK to be stimulated.