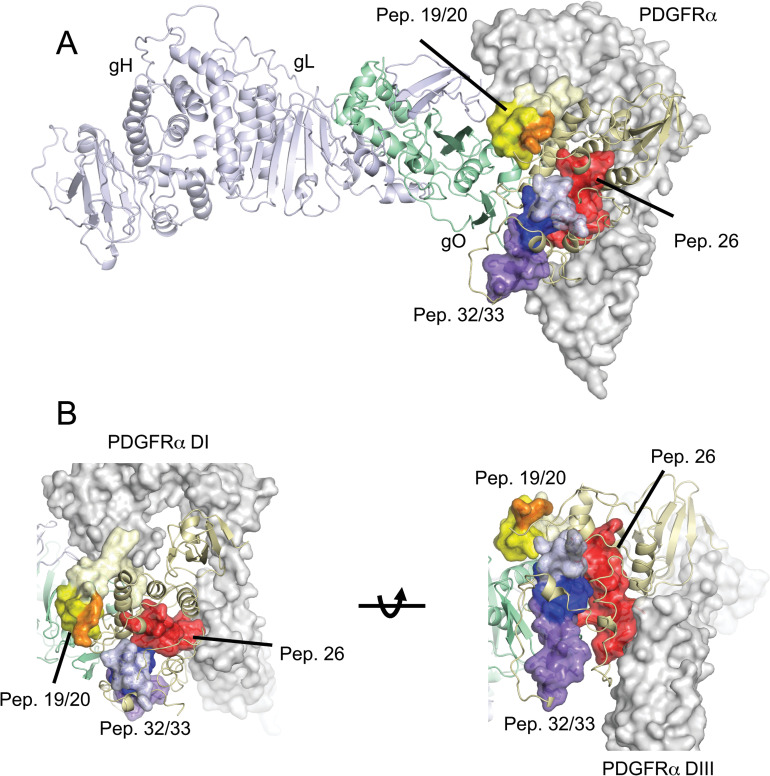
Fig 9. Localization of gO peptides in the structure of trimer bound to PDGFRα.
(A) Peptides 19/20, 26 and 32/33 are shown as colored surfaces on the structure of gHgLAD169gOTR:PDGFRα [40]. Pep19/20 is colored yellow and orange, with the unique residues from pep19 (residues 235–247) colored light yellow, the overlapping residues in peptides 19 and 20 were colored bright yellow (residues 248–254) and the unique pep20 residues colored orange. Note that the C-terminal portion of pep20 (residues 256–267) is in a flexible loop that is not visible in the cryo-EM structure. Pep32/33 is colored blue and purple. The unique residues of pep32 (residues 404–416) are colored light blue, the overlapping residues in pep32 and pep33 are colored dark blue and the residues unique to pep33 are colored purple. Pep26 is colored red. The gH/gL/gO trimer is shown in cartoon format, with gH colored light blue, gL colored light green and gO colored light yellow. PDGFRα is shown as a semitransparent surface colored grey. Panel B shows two different closeup orientations of the gO peptides relative to PDGFRα. The N-terminal region of pep19 makes contacts with PDGFRα DI.