Abstract
Studies have suggested that B vitamins or omega-3 polyunsaturated fatty acids (PUFAs) may deter the development of cardiovascular disease (CVD). This systematic review aims to examine whether the combined supplementation of both B vitamins and omega-3 PUFAs could provide additional beneficial effects to prevent CVD beyond the effect of each supplement based on clinical trials published up to December 2021. The overall findings are inconsistent and inconclusive, yet the combined supplementation of these two nutrients may be more effective at reducing plasma homocysteine, triglyceride, and low-density lipoprotein-cholesterol than the individual components. The underlying mechanisms mainly include alleviating endothelial dysfunction, inhibiting atherosclerosis and lesion initiation, reducing oxidative stress, suppressing activation of pro-inflammatory cytokines, regulating endothelial nitric oxide synthase, and interfering with methylation of genes that promote atherogenesis. Although biologically plausible, the existing literature is insufficient to draw any firm conclusion regarding whether B vitamins can further enhance the potential beneficial effects of omega-3 PUFA intake on either primary or secondary prevention of CVD. The inconsistent findings may be largely explained by the methodological challenges. Therefore, well-designed high-quality trials that will use the combined supplementation of B vitamins and omega-3 PUFAs or dietary patterns rich in these two types of nutrients are warranted.
Keywords: B vitamins, cardiovascular disease, omega-3 PUFAs, supplementation
1. Introduction
Dietary intake of B vitamins or omega-3 polyunsaturated fatty acids (PUFAs) has been found to be inversely related to cardiovascular disease (CVD) [1,2]. The potential mechanisms behind this association are attributed to their roles in lowering the risk factors of CVD, including plasma homocysteine (Hcy), circulating triglycerides (TG) and C-reaction protein (CRP) [1,3,4,5]. These risk factors have been implicated in the atherosclerotic process [6] and endothelial dysfunction [7] which involve in CVD development, therefore, B vitamins and omega-3 PUFAs are expected to contribute to CVD prevention [8,9].
Nevertheless, a recent meta-analysis including 15 randomized controlled trials (RCTs) has found that B vitamins supplementations (vitamin B6, folate, or vitamin B12 given alone or in combination) have little impact on CVD prevention [10], although the other meta-analysis including five RCTs has showed a benefit for folic acid in CVD reduction [11]. Additionally, a meta-analysis including 79 RCTs also reported little to no effect of increasing omega-3 PUFAs intake (particularly in the form of supplements) on CVD prevention [12].
However, results from several clinical trials revealed a potential synergetic influence of B vitamins and omega-3 PUFAs to prevent CVD. In volunteers with normal or slightly to moderately increased blood lipids, supplementation of both B vitamins (vitamin B6 and folic acid) and fish oil for 4 weeks reduced the atherogenic index by 24%, which was greater than the decrease (12%) from fish oil supplementation alone [12]. Similarly, supplementation of both vitamin B12 and fish oil for 8 weeks lowered plasma Hcy concentration more than individual supplementation with either vitamin B12 or fish oil in healthy participants [3]. Since omega-3 PUFA and B vitamins are both involved in metabolism of one carbon units [6,13,14], alterations in each of these nutrients may influence Hcy concentration, methylation reactions and oxidative stress, all of which are involved in the pathological development of CVD [13]. Thus, co-ingesting these two nutrient groups may provide more beneficial effects to prevent CVD, compared to supplements of either B vitamins or omega-3 PUFAs alone.
Therefore, this review aims to examine whether the combined supplementation of B vitamins and omega-3 PUFAs could provide additional beneficial effects on improving risk factors to prevent CVD beyond the effects of either of them alone and delineate any inconsistencies and limitations of the current intervention studies, through comprehensive analysis of the available literature, and, lastly, provide suggestions for future research and dietetic practice or advice.
2. Methods
2.1. Search Strategy
We conducted the literature search on PubMed, EmBase, and the Cochrane Register of Controlled Trials up to December 2021 following the statement checklist of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [15]. The current review was registered with PROSPERO, the international prospective register of systematic reviews (https://www.crd.york.ac.uk/prospero/, accessed on 1 December 2021), with the registration number CRD42018085993. Search terms included the word combinations of the following Medical Subject Headings: [“cardiovascular disease” or “cardiovascular disease risk factor” or “low-density lipoprotein cholesterol” or “LDL” or “C-reactive protein” or “CRP” or “triglyceride” or “TG” or “high-density lipoprotein cholesterol” or “HDL”] AND [“B vitamins” or “vitamin B2” or “riboflavin” or “vitamin B6” or “pyridoxine” or “folate” or “folic acid” or “vitamin B12” or “cobalamin” or “homocysteine” or “Hcy”] AND [“fish oil” or “n-3 fatty acids” or “omega-3 fatty acids” or “docosahexaenoic acid” or “DHA” or “eicosapentaenoic acid” or “EPA’’ or “alpha-linolenic acid” or “ALA”]. Reference lists of all identified articles and recently published reviews were also examined for the search terms.
2.2. Inclusion and Exclusion Criteria
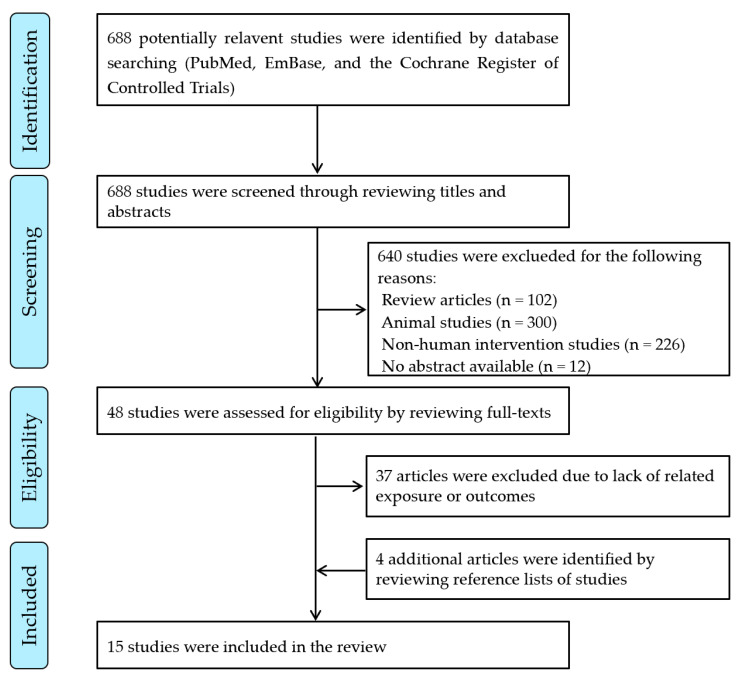
Eligible studies were included in this review if they reported the synergistic effect of the combined supplementation of B vitamins (vitamin B2, vitamin B6, folic acid and vitamin B12) and fish oil (or an omega-3 PUFA term) on risk of CVD and/or its risk factors in humans. No selection restrictions were placed on investigations with respect to study type, study design, publication year or language (if an English abstract was provided). A large number of relevant publications were identified; however, the majority were excluded because they only reported the influence of either B vitamins or fish oil (or omega-3 PUFA) supplementation alone on the prevention or treatment of CVD. Thus, only studies investigating a synergistic effect of fish oil (or fatty acids) and B vitamins in humans were included. Finally, eligible articles were identified and assessed by one author (J.Z.). A second researcher (P.C.X.) independently evaluated and verified inclusion/exclusion decisions. Study selection and screening process shown in Figure 1.
Figure 1.
Flow diagram of identification, screening, and selection process for included articles.
2.3. Data Extraction and Study Quality Evaluation
Data were extracted from the original studies, which included first author name, the publication year, study location/design/duration, participants information, intervention, and outcomes. The quality criteria checklist of the Evidence Analysis Manual (2016) of the Academy of Nutrition and Dietetics was applied to evaluate study quality and risk of bias [16].
3. Results
The search yielded a total of 688 potentially relevant articles, of which 640 were excluded because they are review articles or animal studies or non-human intervention studies or with no abstract available; 48 full-text publications were retrieved and obtained for detailed review, of which 37 were excluded due to lack of related exposure or outcomes, and 4 additional articles were identified and included after reviewing the reference lists. Following detailed review, 15 studies met the inclusion criteria [3,4,5,6,12,14,17,18,19,20,21,22,23,24,25], i.e., reporting the synergistic effect of the combined supplementation of B vitamins (vitamin B2, vitamin B6, folic acid, and vitamin B12) and fish oil (or omega-3 fatty acids), or together with other nutrients in humans (Figure 1).
As shown in Table 1, the sample sizes ranged from 12 to 2501 participants with study duration ranging from 4 weeks to 4.7 years. Additionally, the dose ranges of the B vitamins or omega-3 PUFAs differed dramatically [vitamin B6 (2.5–80) mg/day, vitamin B12 (20–1000) μg/day, folic acid (150–10,000) μg/day and omega-3 PUFAs (0.2–2) g/day]. Fourteen studies [3,4,5,12,14,17,18,19,20,21,22,23,24,25] investigated the effects of the combined supplementation of B vitamins and omega-3 PUFAs on blood Hcy with most studies reporting Hcy-lowering effect [3,4,5,12,14,17,19,20,21,22,23,24,25]. The effects of the above combined nutrients on CRP were examined by eight trials, three of which reported beneficial effects [3,19,22] with insignificant changes in CRP values found in the other studies [4,5,18,20,21]. Among eleven trials investigating the impact of the combined supplementation of B vitamins and omega-3 PUFAs on plasma TG [3,4,5,12,17,18,19,20,21,22,24,25], seven studies reported the lowering effects of TG upon joint consumption of B vitamins and omega-3 PUFAs [3,4,12,18,19,21,25], while the four remaining trials demonstrated no change in plasma TG [5,20,22,24]. Additionally, seven trials investigated the impact of the combined administration of B vitamins and omega-3 PUFAs on plasma low-density lipoprotein cholesterol (LDL-C) with six studies [5,18,21,22,24,25] showing decreasing LDL-C effects and the other one reporting no obvious alteration of LDL-C concentration [23]. Moreover, five studies assessed the influence of the joint supplementation of B vitamins and omega-3 PUFAs on plasma high-density lipoprotein cholesterol (HDL-C) with elevating effects observed in two trials [12,18] and no changes reported in other three studies [5,22,24]. There is no intervention study included in this review reporting the effect of the combined B vitamins and omega-3 PUFAs administration on primary end points of CVD, because the trails were either conducted in participants with cardiovascular disease at baseline [6,14,17,22] or cardiovascular risk factors were only assayed in the studies [3,4,5,12,18,19,20,21,23,24,25].
Table 1.
Characteristics of the studies included in the systematic review.
| Reference | Year | Location | Participants | Design | Intervention | Duration | Outcomes |
|---|---|---|---|---|---|---|---|
| Haglund et al. [12] | 1993 | Sweden | n = 12, male volunteers, healthy or with slightly to moderately increased blood lipids | A double-blind cross-over study | 30 mL of fish oil with 30 mL of orange juice/day for 4 weeks followed by a 5-week washout, and then 30 mL of fish oil with 30 mL of orange juice/day supplemented with vitamin B6 (80 mg/day) and folic acid (10 mg/day) for 4 weeks. Fish oil contained 13% DHA and 19% EPA. |
Fish oil- supplemented orange juice for 4 weeks followed by a 5-week washout period and then B vitamins + fish oil-supplemented orange juice for 4 weeks |
|
| Baró et al. [24] | 2003 | Spain | n = 30, healthy volunteers (50% man) | CS without control | 500 mL/day of semi-skimmed milk for 4 weeks and then 500 mL/day of the enriched milk (containing omega-3 PUFAs, oleic acid, vitamins E, B6, and folic acid) for 8 further weeks. | 8 weeks |
|
| Carrero et al. [26] | 2004 | Spain | n = 30, volunteers (50% man) with mild hyperlipidemia | CS without control | 500 mL/day of semi-skimmed milk for 4 weeks and then 500 mL/day of the enriched milk (containing omega-3 PUFAs, oleic acid, vitamins E, B6, and folic acid) for 8 weeks. | 8 weeks |
|
| Carrero et al. [20] | 2005 | Spain | n = 60, patients with PVD and intermittent claudication (100% man) | RCT with parallel- group design |
Supplemented group: 500 mL/day of a fortified dairy product containing EPA, DHA, oleic acid, folic acid, and vitamins B6, D, A, and E; Control group: 500 mL/day of semi-skimmed milk with added vitamins A and D. |
12 months |
|
| Benito et al. [21] | 2006 | Spain | n = 72, patients with metabolic syndrome | A randomized, placebo- controlled and open clinical trial of parallel design |
Control group: 500 mL semi-skimmed milk/day; Test group: 36 patients consumed 500 mL enriched milk (5.7 g of oleic acid, 0.2 g of omega-3 PUFAs, 150 μg of folic acid and 7.5 mg of vitamin E) /day. |
3 months |
|
| Carrero et al. [23] | 2006 | Spain | n = 40, male PVD patients | A longitudinal, randomized, controlled study | Group 1: 500 mL/day fortified dairy product containing fish oil, oleic acid, folic acid, vitamins A, D, E, and B6; Group 2: 500 mL/day fortified dairy product + simvastatin (20 mg/day); Group 3: 500 mL/day semi-skimmed milk; Group 4: 500 mL/day semi-skimmed milk + simvastatin (20 mg/day). |
12 months |
|
| Carrero et al. [22] | 2007 | Spain | n = 40, patients suffered from MI (100% man) | RCT with parallel- group design |
Supplemented group: 500 mL/day of a fortified dairy product containing EPA, DHA, oleic acid, folic acid, and vitamins B6, D, A, and E; Control group: 500 mL/day of semi-skimmed milk with added vitamins A and D. |
12 months |
|
| Fonollá et al. [18] | 2009 | Spain | n = 297, participants (84.5% man) with moderate cardiovascular risk | RCT with parallel- group design |
Group 1: 500 mL/day enriched milk (containing omega-3 PUFAs, oleic acid, vitamins E, B6, and folic acid); Group 2: 500 mL/day skimmed milk; Group 3 (control): 500 mL/day semi-skimmed milk. |
12 months |
|
| Galan et al. [14] | 2010 | France | n = 2501 patients (79.5% man) with CVD (MI, stroke and unstable angina) | RCT with a 2 × 2 factorial design | Group 1: B-vitamins [5-methyl-THF (560 μg), vitamin B6 (3 mg) and vitamin B12 (20 μg)] and a placebo capsule for omega-3 PUFAs; Group 2: omega-3 PUFAs (600 mg of EPA and DHA at a ratio of 2:1) and a placebo capsule for B-vitamins; Group 3: both B-vitamins and omega-3 PUFAs; Group 4: placebo capsules for both treatments. |
4.7 years |
|
| Szabo et al. [17] | 2012 | France | n = 2501, patients (79.5% man) with a history of CVD (MI, stroke and unstable angina) | RCT with a 2 × 2 factorial design | Group 1: B-vitamins [5-methyl-THF (560 μg), vitamin B6 (3 mg) and vitamin B12 (20 μg)] and a placebo capsule for omega-3 PUFAs; Group 2: omega-3 PUFAs (600 mg of EPA and DHA at a ratio of 2:1) and a placebo capsule for B-vitamins; Group 3: both B-vitamins and omega-3 PUFAs; Group 4: placebo capsules for both treatments. |
4.7 years |
|
| Earnest et al. [19] | 2012 | UK | n = 100, participants with elevated Hcy (>8.0 umol/L) | RCT with 2 × 2 factorial design | Group 1: placebo; Group 2: MVit (vit C: 200 mg; vitE: 400 IU; vit B6: 25 mg; folic acid: 400 ug; vit B12: 400 ug) + placebo; Group 3: omega-3 PUFAs (2 g omega-3 PUFAs, 760 mg EPA, 440 mg DHA) + placebo; Group 4: MVit + omega-3 PUFAs. |
12 weeks |
|
| De Natale C et al. [4] | 2012 | Italy | n = 16, participants (43.8% man) with mild plasma lipid abnormalities | a randomized crossover design | Group 1: a diet containing baked products enriched with active nutrients [β-glucans (3.6 g/day), folic acid (1620 μg/day), long-chain (800 mg/day) and short-chain (400 mg/day) omega-3 PUFAs, and tocopherols (120 mg/day); Group 2: a diet containing the same products without active nutrients (control diet). |
1 month for each of the control and enriched diets and then cross over to the other diet |
|
| Blacher et al. [6] | 2013 | France | n = 2501, patients (79.5% man) with a past history of cardio- or cerebrovascular diseases | RCT with a 2 × 2 factorial design | Group 1: B-vitamins [(5-methyl-THF (560 μg), vitamin B6 (3 mg) and vitamin B12 (20 μg)] and a placebo capsule for omega-3 PUFAs; Group 2: omega-3 PUFAs (600 mg of EPA and DHA at a ratio of 2:1) and a placebo capsule for B-vitamins; Group 3: both B-vitamins and omega-3 PUFAs; Group 4: placebo capsules for both treatments. |
4.2 (± 1.0) years |
|
| Garaiova et al. [5] | 2013 | Slovakia | n = 25, hypercholesterolmic children and adolescents, mean age 16.4 ± 3.8 years | CS without control | A combination of plant sterols esters (1300 mg), fish oil (1000 mg EPA plus DHA) and vitamins B12 (50 μg), B6 (2.5 mg), folic acid (800 μg) and coenzyme Q10 (3 mg). | 16 weeks |
|
| Huang et al. [3] | 2015 | China | n = 38, healthy individuals, 57% man, 23 ± 3 years of old | RCT with parallel- group design |
Group 1: vitamin B12 (1000 μg); Group 2: fish oil (2 g); Group 3: vitamin B12 (1000 μg) + fish oil (2 g) (each 1 g capsule provided 490 mg of 22:6 omega-3, and 98 mg of 20:5 omega-3). |
8 weeks |
|
PUFAs, polyunsaturated fatty acids; CVD, cardiovascular diseases; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; Hcy, homocysteine; CS, cross-sectional study; LDL-C, low-density lipoprotein cholesterol; VCAM-1, vascular cell adhesion molecule-1; MDA, malondialdehyde; PVD, peripheral vascular disease; RCT, randomized controlled trial; ApoB, apolipoprotein B; CRP, C-reaction protein; PAI-1, plasminogen activator inhibitor-1; ICAM-1, intercellular adhesion molecule-1; ABI, ankle-brachial pressure index; MI, myocardial infarction; THF, tetrahydrofolic acid; HR, hazard ratio; CI, confidence interval; BP, blood pressure; MVit, multivitamins; OR, odds ratio; VLDL-C, very low-density lipoprotein cholesterol; ↓, decrease; ↑, increase.
For all included literature, 4 studies [19,21,24,25] received positive scores and 11 studies [3,4,5,6,12,14,17,18,20,22,23] received neutral scores by using the published format [26] based on the quality criteria checklist (Supplemental Table S1).
4. Discussion
Although several studies have demonstrated that the combined supplementation of B vitamins and omega-3 PUFAs has diverse effects on the CVD risk factors, it is insufficient to draw any firm conclusion as to whether B vitamins can further enhance the potential beneficial effects of omega-3 PUFA intake on either primary or secondary prevention of CVD.
4.1. B Vitamins and Omega-3 PUFAs Supplementation and CVD Risk Factors
Hyperhomocysteinemia is recognized as an independent risk factor of CVD [3,6,27]. Hcy can be either metabolized to cysteine or recycled to methionine with the aid of a group of B vitamins, through roles as essential coenzymes (vitamin B2, B6 and B12) or as an essential substrate donor (folate) in one-carbon unit metabolism [8]. It has been well established that supplementation with B vitamins efficaciously lowers plasma Hcy due to direct participation in degradation of Hcy [28,29].
Omega-3 PUFAs can originate from the plant sources, i.e., alpha-linolenic acid (ALA), and the more elongated and desaturated fatty acids from animal sources, i.e., eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [30]. Omega-3 PUFAs are the major fatty acids found in cholesteryl esters and phospholipids, which constitutes to the essential membrane component of cells and intracellular organelles [8,30]. However, the impact of omega-3 PUFAs supplementation on plasma Hcy remains elusive. Some studies have shown that omega-3 PUFAs consumption reduced plasma Hcy levels [3,31], whereas a significant decrease in plasma Hcy was not observed in other similar studies [18,32] These conflicting results may be due to interventions that differed in duration and used different types of participant populations [3].
Moreover, it was found that the combined supplementation of vitamin B12 (1000 μg) and fish oil (2 g, containing 980 mg DHA and 196 EPA) for 8 weeks was more effective at lowering plasma Hcy concentration than either vitamin B12 or fish oil supplementation alone in healthy participants [3]. Likewise, consumption of 500 mL/day milk (containing omega-3 PUFAs [EPA 1.4% and DHA 2.1% in milk fat], oleic acid [54.4% in milk fat], vitamins E [1.50 mg/100 mL], B6 [0.30 mg/100 mL], and folic acid [30 mg/100 mL]) decreased plasma Hcy levels in healthy participants [24], volunteers with mild hyperlipidemia [25], patients with peripheral vascular disease (PVD) [20], patients with metabolic syndrome (MS) [21], and participants suffering from myocardial infarction (MI) [22]. Additionally, a baked functional food product supplemented with sterols esters (1300 mg), fish oil (1000 mg EPA plus DHA), vitamins B12 (50 μg), B6 (2.5 mg), folic acid (800 μg), and coenzyme Q10 (3 mg) that was administered in a balanced diet decreased fasting Hcy in patients with mild-mixed hyperlipidemia [5]. A recent meta-analysis also suggested that a combination of omega-3 PUFAs (0.2–6.0 g/day), folic acid (150–2500 μg/day), and vitamins B6 and B12 appeared to be superior at reducing plasma Hcy than omega-3 PUFAs supplementation alone [27].
CRP is another independent risk predictor for CVD as a biomarker of systemic inflammation [22,33]. In this review, eight trials investigated the effects of the combined intake of B vitamins and omega-3 PUFAs on CRP (mainly in the form of high sensitivity CRP) [3,4,5,18,19,20,21,22]. Three studies reported beneficial effects on CRP [3,19,22], while the other studies failed to find significant changes in CRP values following the joint supplementation [4,5,18,20,21]. Huang et al. showed that administration of fish oil and vitamin B12 significantly reduced plasma CRP levels, compared to the values of the participants at baseline [3]. Consistent with this trial, Carrero et al. demonstrated that high plasma CRP concentrations declined in MI patients who consumed fortified semi-skimmed milk supplemented with omega-3 PUFAs, oleic acid, B vitamins (folic acid and vitamins B6), and vitamin E [22], whereas De Natale et al. showed that this supplemented dairy product failed to change CRP values either in patients with MS [4] or patients with PVD [20]. Additionally, no remarkable alterations in CRP values were observed in patients with mild mixed hyperlipidemia [4], hypercholesterolemic children and adolescents [5], and participants with moderate cardiovascular risk [18], following consumption of the test products containing B vitamins and omega-3 PUFAs. The contradictory findings may be due to initial metabolic differences in the study populations, as participants had quite low CRP values in one study at baseline [4] and a more significant beneficial effect may be observed in individuals with higher initial levels, such as patients with diabetes [4]. Moreover, other than different doses and combinations of B vitamins and omega-3 PUFAs administered in trials, the addition of other nutrients, such as vitamin C or oleic acid, to the supplementation may have altered the synergistic effect between B vitamins and omega-3 PUFAs on CRP [19,22].
TG and LDL-C were positively associated with the incidence of CVD [3,4,5,21,25]. Single supplemental omega-3 PUFAs could decrease TG [34], which were commonly observed in adults with hyperlipidemia when it was supplemented at higher doses (ranging from 2 to 4 g/day) [35,36,37]; however, these results were not observed in adults with normal lipid levels, and effects of supplemental omega-3 PUFA on LDL-C and HDL-C remain inconsistent. [21,38,39,40,41,42]. More recent studies investigating specific omega-3 PUFA combinations have shown that reduction in TG levels in adults with high cholesterol are observed with animal-based EPA + DHA supplementation, sole EPA supplementation derived from either fish or microalgae, but not with sole ALA supplementation [43,44]. Additionally, single supplemental B vitamins have also been shown to reduce TG and cholesterol in adults with heart diseases [28].
In the studies included in the present review, the various dosages and combinations of the nutrients may contribute to the heterogeneous effects of the combined supplementation of B vitamins and omega-3 PUFAs on TG, LDL-C, and HDL-C. Furthermore, the variations in LDL-C are not concomitant with the changes of TG under co-ingestion of B vitamins and omega-3 PUFAs [5,22,24]. The extra ingredients contained in the fortified food may also counteract the effect exerted by the combined supplementation of B vitamins and omega-3 PUFAs on LDL-C. In the study by De Natale et al., the enriched functional products decreased plasma TG, but did not change plasma LDL-C [4]. This may be due to β-glucan supplemented to the product food, which has the properties of effectively lowering cholesterol and, thus, maybe counterbalancing the untoward impact of omega-3 PUFAs on LDL-C [4]. Additionally, the daily intake of the enriched milk fortified with folic acid and omega-3 PUFAs resulted in both plasma LDL-C and TG reduction in patients with mild hyperlipidemia [25], participants with moderate cardiovascular risk [18], and patients with MS [21], but only led to LDL-C reduction with no changes in TG in healthy participants [22] and patients suffering from MI [23]. The possible reasons for these disputed results may be attributed to various health statuses in participants and different study designs.
4.2. B Vitamins and Omega-3 PUFAs Supplementation and CVD Risk
There was lack of consistent beneficial effects of B vitamins supplementation on CVD risk. This is supported by a meta-analysis including 15 RCTs which showed that null effect was found under B-complex vitamin therapy (vitamin B6, folate, or vitamin B12 given alone or in combination) on CVD prevention [10]. However, another recent meta-analysis demonstrated that both folic acid (7 RCTs) and B-complex vitamins (12 RCTs) reduced incident stroke [45]. Similarly, the absence of consistent beneficial effects of omega-3 PUFAs consumption on the rate of CVD was also observed. A large meta-analysis including 79 RCTs with 12 to 72 months’ duration indicated that increasing intake of EPA and DHA (mainly from supplementation) exerted little or no influence on mortality or cardiovascular health, whereas ALA consumption might slightly decrease CVD risk [34]. Nevertheless, a recent updated meta-analysis including RCTs reported that supplemental marine omega-3 FAs decreased coronary risk [46].
Moreover, there was no study included in the present review reporting the effect of the combined supplementation of B vitamins and omega-3 PUFAs on primary prevention of CVD. Regarding secondary CVD endpoints, the SU.FOL.OM3 trial involving 2501 patients with a history of CVD (MI, stroke, and unstable angina) treated with B vitamins (5-methyl-tetrahydrofolic acid + vitamin B6 + vitamin B12) and/or omega-3 PUFAs for about 4.7 years, had reported neither B vitamins nor omega-3 PUFAs significantly decreased risks of hard coronary events as well as coronary revascularization [6]. This trial also reported that neither B vitamins nor omega-3 PUFAs supplementation exerted effects on blood pressure and the rates of other cardiovascular events in patients with a history of established coronary or cerebrovascular diseases [14,17]. Since this RCT with a double-blind placebo-controlled 2 × 2 factorial design, the findings are relatively reliable.
4.3. Possible Mechanisms
B vitamins and DHA are interlinked in one-carbon unit metabolism [47,48]. An animal study revealed that the addition of fish oil to a vitamin B6 insufficient diet may prevent the reduced synthesis of EPA and DHA [49]. Likewise, insufficient folic acid in diet significantly reduced omega-3 PUFAs in plasma and platelets, compared to the rats fed with adequate folate [8,50]. The possible explanation may be due to the enhanced Hcy level induced by folate depletion, which, in turn, contributes to Hcy-associated oxidative stress with more PUFAs oxidized [8,51]. Therefore, alterations in each of the above nutrients can influence Hcy homeostasis, oxidative stress status and methylation reactions [13]. Furthermore, it has been proved that Hcy can induce atherosclerosis through promoting lipoprotein oxidation and endothelial dysfunction, as well as increasing cholesterol synthesis [25]. The increased reactive oxygen species are activators of the transcription factors, such as nuclear factor kappa B (NF-κB) [50]. The Hcy-stimulated NF-κB can lead to a pleiotropic response involving up-regulation of endothelial activation factors [47], contributing to early atherosclerosis and lesion initiation [24]. NF-κB activation is also a potent inducer of proinflammatory cytokine [52]. Therefore, B vitamins and omega-3 PUFAs may protect against endothelial dysfunction via reduction of proinflammatory cytokines activation through anti-hyperhomocysteinemia and lowering oxidative stress [7,13]. Alternatively, omega-3 PUFAs are postulated to inhibit lipogenesis and enhance resolvin and protectin generation, eventually contributing to decreased inflammation [53]. Possible direct adverse effects on atherosclerosis could also appear under high doses of B vitamins, such as folic acid [4]. The possible mechanisms may be because folic acid promotes the remethylation of Hcy to methionine with elevating S-adenosyl methionine, thus resulting in enhancing asymmetrical dimethylarginine concentrations, which may suppress the synthase of endothelial nitric oxide [8,54]. Additionally, these folic acid-induced methylation effect may also influence several proatherogenic genes expression [54].
4.4. Implications for Practice
Although the combined supplementation of B vitamins and omega-3 PUFAs may have more beneficial impact on CVD prevention than B vitamins or omega-3 PUFAs given alone, dietetic strategies for preventing CVD need to focus more on the importance of considering effects at the whole food and dietary patterns level. Healthy dietary patterns including foods rich in B vitamins and omega-3 PUFAs (such as fish, vegetable, fruit, legumes, nuts, and eggs) are likely more useful for preventing or treating CVD, because they provide other beneficial nutrients which are not present in the pure supplements [55,56]. The focus of primary prevention remains rooted in a whole diet approach. For example, the Mediterranean diet, which is rich in omega-3 PUFAs and B vitamins, was recently reported to reduce CVD risk by 20–25% in individuals with high adherence to this diet [57]. The synergistically beneficial effects on CVD risk from the possible interactions among multiple nutrients in foods abundant in B vitamins and omega-3 PUFAs cannot be ruled out.
4.5. Study Limitations and Methodology Challenges
Several limitations need to be considered when interpreting the findings from this review. First, the regimens of nutrient supplementation are different among the human trials, which include capsules, an enriched bake product, an emulsified preparation, and enriched milk with different dosages and combinations of B vitamins and omega-3 PUFAs. Additionally, although the capsules allocated to different groups were identical in appearance and smell, as well as taste, the number of total capsules to be taken in Huang et al.’s study was different among groups [3]. Therefore, potential bias may exist, and techniques, such as double dummy, are suggested in future studies to avoid such bias. Second, owing to the absence of randomization and/or a placebo-controlled group in some included trials, the so-called “placebo effect” cannot be excluded. Furthermore, without the placebo-controlled group, interaction effect could not be determined. Therefore, a factorial study design, e.g., 2 × 2 factorial design, is warranted for future studies, which allows the researchers to investigate the interaction between B vitamins and omega-3 PUFAs in addition to their respective main effects. Third, participants in most of the included trials maintained the habitual diet. There was no information about the amount of B vitamins and of omega-3 PUFAs intake in the usual diet. Fortification status with folic acid in different regions may also influence the effect of the above nutrients’ supplementation on CVD [58]. Thus, the variation in diet could introduce residual confounding that might bias the results of the combined supplementation in the trials especially in the small-scale trials. Fourth, because the participants with CVD received up-to-date pharmacological treatments with nutrient supplementation as an auxiliary means, cardiovascular risk might be further decreased, which possibly masked the potential effects of supplemental B vitamins and omega-3 PUFAs [5]. Fifth, there were significant differences in intervention duration, the dosage and formation of the combined supplements across the included studies, which might lead to the inconsistent findings. Moreover, the potential effects of genetic risk factors influencing B vitamins and omega-3 PUFAs metabolism were not examined in the included studies. Genetic variations encoding the key enzymes may affect individual’s capacity to utilize/metabolize nutrients [59], thus influencing efficacy of B vitamins and n-3 LC-PUFA supplementation on CVD prevention among diverse population. Additionally, the possible limitations of the present review itself (such as search strategy) cannot be completely ruled out.
5. Conclusions
The limited evidence from intervention studies indicates that the combined supplementation with B vitamins and omega-3 PUFAs may be promising and more effective at reducing plasma Hcy, TG, and LDL-C than each supplementation alone. However, there is no solid evidence that the joint supplementation of these two can offer a synergistic effect on preventing CVD and decreasing the relevant morbidity and/or mortality in susceptible populations. Due to the methodological challenges and heterogeneity in study design of the existing trials, it is difficult to draw any definitive conclusions based on the current literature. Therefore, well-designed high-quality trials that will use the combined supplementation of B vitamins and omega-3 PUFAs or dietary patterns rich in these two types of nutrients are warranted.
Abbreviations
ALA, alpha-linolenic acid; CRP, C-reaction protein; CVD, cardiovascular diseases; Hcy, homocysteine; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MS, metabolic syndrome; MI, myocardial infarction; PUFAs, polyunsaturated fatty acids; PVD, peripheral vascular disease; RCTs, randomized controlled trials; TG, triglyceride.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14081608/s1, Table S1: Research validity assessment using the American Dietetic Association’s Quality Criteria Checklist.
Author Contributions
K.K. conceived the study concept, designed the protocol, and supervised the manuscript writing; J.Z. and P.-C.X. conducted literature search and review, performed study selection and data extraction, and drafted the manuscript; J.Z., P.-C.X., M.K., K.-F.Y., A.D.F. and K.K. critically reviewed the manuscript for important intellectual content, and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by National Institutes of Health National Institute of Environmental Health Sciences grant R01ES021735 and Texas State University Research Enhancement Program 2022 and the Subvention Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He K., Merchant A., Rimm E.B., Rosner B.A., Stampfer M.J., Wllett W.C., Ascherio A. Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35:169–174. doi: 10.1161/01.STR.0000106762.55994.86. [DOI] [PubMed] [Google Scholar]
- 2.Hu F.B., Bronner L., Willett W.C., Stampfer M.J., Rexrode K., Albert C., Hunter D., Manson J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 3.Huang T., Li K., Asimi S., Chen Q., Li D. Effect of vitamin B-12 and n-3 polyunsaturated fatty acids on plasma homocysteine, ferritin, C-reaction protein, and other cardiovascular risk factors: A randomized controlled trial. Asia Pac. J. Clin. Nutr. 2015;24:403–411. doi: 10.6133/apjcn.2015.24.3.19. [DOI] [PubMed] [Google Scholar]
- 4.De Natale C., Minerva V., Patti L., Mazzarella R., Ciano O., Maione S., Luongo D., Naviglio D., Marotta G., Turco S., et al. Effects of baked products enriched with n-3 fatty acids, folates, beta-glucans, and tocopherol in patients with mild mixed hyperlipidemia. J. Am. Coll. Nutr. 2012;31:311–319. doi: 10.1080/07315724.2012.10720427. [DOI] [PubMed] [Google Scholar]
- 5.Garaiova I., Muchová J., Nagyová Z., Mišľanová C., Oravec S., Dukát A., Wang D., Plummer S.F., Ďuračková Z. Effect of a plant sterol, fish oil and B vitamin combination on cardiovascular risk factors in hypercholesterolemic children and adolescents: A pilot study. Nutr. J. 2013;12:7. doi: 10.1186/1475-2891-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blacher J., Czernichow S., Paillard F., Ducimetiere P., Hercberg S., Galan P. Cardiovascular effects of B-vitamins and/or N-3 fatty acids: The SU.FOL.OM3 trial. Int. J. Cardiol. 2013;167:508–513. doi: 10.1016/j.ijcard.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.de Bree A., Mennen L.I., Hercberg S., Galan P. Evidence for a protective (synergistic?) effect of B-vitamins and omega-3 fatty acids on cardiovascular diseases. Eur. J. Clin. Nutr. 2004;58:732–744. doi: 10.1038/sj.ejcn.1601871. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y., Lu L., Liang J., Liu M., Li X., Sun R., Zheng Y., Zhang P. Omega-3 Fatty Acids for Primary and Secondary Prevention of Cardiovascular Disease. Cell Biochem. Biophys. 2015;72:77–81. doi: 10.1007/s12013-014-0407-5. [DOI] [PubMed] [Google Scholar]
- 10.Martí-Carvajal A.J., Solà I., Lathyris D., Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017;8:CD006612. doi: 10.1002/14651858.CD006612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins D.J.A., Spence J.D., Giovannucci E.L., Kim Y.I., Josse R.G., Vieth R., Sahye-Pudaruth S., Paquette M., Patel D., Mejia S., et al. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. J. Am. Coll. Cardiol. 2021;77:423–436. doi: 10.1016/j.jacc.2020.09.619. [DOI] [PubMed] [Google Scholar]
- 12.Haglund O., Hamfelt A., Hambraeus L., Saldeen T. Effects of fish oil supplemented with pyridoxine and folic acid on homocysteine, atherogenic index, fibrinogen and plasminogen activator inhibitor-1 in man. Nutr. Res. 1993;13:1351–1365. doi: 10.1016/S0271-5317(05)80786-9. [DOI] [Google Scholar]
- 13.Kemse N.G., Kale A.A., Joshi S.R. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy induced hypertension. PLoS ONE. 2014;9:e111902. doi: 10.1371/journal.pone.0111902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galan P., Kesse-Guyot E., Czernichow S., Briancon S., Blacher J., Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Academy of Nutrition and Dietetics Evidence Analysis Manual. 2016. [(accessed on 21 November 2018)]. Available online: https://www.andeal.org/evidence-analysis-manual.
- 17.De Edelenyi F.S., Vergnaud A.-C., Ahluwalia N., Julia C., Hercberg S., Blacher J., Galán P. Effect of B-vitamins and n-3 PUFA supplementation for 5 years on blood pressure in patients with CVD. Br. J. Nutr. 2012;107:921–927. doi: 10.1017/S0007114511003692. [DOI] [PubMed] [Google Scholar]
- 18.Fonollá J., Lopez-Huertas E., Machado F.J., Molina D., Álvarez I., Mármol E., Navas M., Palacín E., García-Valls M.J., Remón B., et al. Milk enriched with "healthy fatty acids" improves cardiovascular risk markers and nutritional status in human volunteers. Nutrition. 2009;25:408–414. doi: 10.1016/j.nut.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Earnest C.P., Kupper J.S., Thompson A.M., Guo W., Church T. Complementary effects of multivitamin and omega-3 fatty acid supplementation on indices of cardiovascular health in individuals with elevated homocysteine. Int. J. Vitam. Nutr. Res. 2012;82:41–52. doi: 10.1024/0300-9831/a000093. [DOI] [PubMed] [Google Scholar]
- 20.Carrero J.J., López-Huertas E., Salmerón L.M., Baró L., Ros E. Daily supplementation with (n-3) PUFAs, oleic acid, folic acid, and vitamins B-6 and E increases pain-free walking distance and improves risk factors in men with peripheral vascular disease. J. Nutr. 2005;135:1393–1399. doi: 10.1093/jn/135.6.1393. [DOI] [PubMed] [Google Scholar]
- 21.Benito P., Caballero-Villarraso J., Moreno J., Gutiérrez-Alcántara C., Muñoz C., Rojo-Martínez G., Garcia S., Soriguer F.C. Effects of milk enriched with omega-3 fatty acid, oleic acid and folic acid in patients with metabolic syndrome. Clin. Nutr. 2006;25:581–587. doi: 10.1016/j.clnu.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Carrero J.J., Fonollá J., Marti J.L., Jiménez J., Boza J.J., López-Huertas E. Intake of fish oil, oleic acid, folic acid, and vitamins B-6 and E for 1 year decreases plasma C-reactive protein and reduces coronary heart disease risk factors in male patients in a cardiac rehabilitation program. J. Nutr. 2007;137:384–390. doi: 10.1093/jn/137.2.384. [DOI] [PubMed] [Google Scholar]
- 23.Carrero J.J., López-Huertas E., Salmerón L.M., Ramosc V.E., Barób L., Rosc E. Simvastatin and supplementation with ω-3 polyunsaturated fatty acids and vitamins improves claudication distance in a randomized PILOT study in patients with peripheral vascular disease. Nutr. Res. 2006;26:637–643. doi: 10.1016/j.nutres.2006.09.024. [DOI] [Google Scholar]
- 24.Baró L., Fonollá J., Peña J.L., Martínez-Férez A., Lucena A., Jiménez J., Boza J.J., López-Huertas E. n-3 Fatty acids plus oleic acid and vitamin supplemented milk consumption reduces total and LDL cholesterol, homocysteine and levels of endothelial adhesion molecules in healthy humans. Clin. Nutr. 2003;22:175–182. doi: 10.1054/clnu.2002.0620. [DOI] [PubMed] [Google Scholar]
- 25.Carrero J.J., Baró L., Fonollá J., González-Santiago M., Martinez-Ferez A., Castillo R., Jiménez J., Boza J.J., López-Huertas Y.E. Cardiovascular effects of milk enriched with omega-3 polyunsaturated fatty acids, oleic acid, folic acid, and vitamins E and B6 in volunteers with mild hyperlipidemia. Nutrition. 2004;20:521–527. doi: 10.1016/j.nut.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Ji X., Grandner M.A., Liu J. The relationship between micronutrient status and sleep patterns: A systematic review. Public Health Nutr. 2017;20:687–701. doi: 10.1017/S1368980016002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almassinokiani F., Kashanian M., Akbari P., Mossayebi E., Sadeghian E. Folic acid supplementation reduces plasma homocysteine in postmenopausal women. J. Obstet. Gynaecol. 2016;36:492–495. doi: 10.3109/01443615.2015.1091811. [DOI] [PubMed] [Google Scholar]
- 28.Dawson S.L., Bowe S.J., Crowe T.C. A combination of omega-3 fatty acids, folic acid and B-group vitamins is superior at lowering homocysteine than omega-3 alone: A meta-analysis. Nutr. Res. 2016;36:499–508. doi: 10.1016/j.nutres.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Liu M., Wang Z., Liu S., Liu Y., Ma Y., Liu Y., Xue M., Li Q., Zhang X., Zhang S., et al. Effect of B vitamins supplementation on cardio-metabolic factors in patients with stable coronary artery disease: A randomized double-blind trial. Asia Pac. J. Clin. Nutr. 2020;29:245–252. doi: 10.6133/apjcn.202007_29(2).0006. [DOI] [PubMed] [Google Scholar]
- 30.Micronutrient Information Center, Oregon State University Essential Fatty Acids. 2014. [(accessed on 27 December 2021)]. Available online: http://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids.
- 31.Pooya S.H., Jalali M.D., Jazayery A.D., Saedisomeolia A., Eshraghian M.R., Toorang F. The efficacy of omega-3 fatty acid supplementation on plasma homocysteine and malondialdehyde levels of type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2010;20:326–331. doi: 10.1016/j.numecd.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Grundt H., Nilsen D.W., Hetland O., Mansoor M.A., Aarsland T., Woie L. Atherothrombogenic risk modulation by n-3 fatty acids was not associated with changes in homocysteine in subjects with combined hyperlipidaemia. Thromb. Haemost. 1999;81:561–565. doi: 10.1055/s-0037-1614524. [DOI] [PubMed] [Google Scholar]
- 33.Sara J.D., Prasad M., Zhang M., Lennon R.J., Herrmann J., Lerman L.O., Lerman A. High-sensitivity C-reactive protein is an independent marker of abnormal coronary vasoreactivity in patients with non-obstructive coronary artery disease. Am. Heart J. 2017;190:1–11. doi: 10.1016/j.ahj.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 34.Abdelhamid A.S., Brown T.J., Brainard J.S., Biswas P., Thorpe G.C., Moore H.J., Deane K.H., AlAbdulghafoor F.K., Summerbell C.D., Worthington H.V., et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018;7:CD003177. doi: 10.1002/14651858.CD003177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin Y., Zhou Y., Chen S.-H., Zhao X.-L., Ran L., Zeng X.-L., Wu Y., Chen J.-L., Kang C., Shu F.-R., et al. Fish Oil Supplements Lower Serum Lipids and Glucose in Correlation with a Reduction in Plasma Fibroblast Growth Factor 21 and Prostaglandin E2 in Nonalcoholic Fatty Liver Disease Associated with Hyperlipidemia: A Randomized Clinical Trial. PLoS ONE. 2015;10:e0133496. doi: 10.1371/journal.pone.0133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S.Y., Mayneris-Perxachs J., Lovegrove J.A., Todd S., Yaqoob P. Fish-oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am. J. Clin. Nutr. 2014;100:1232–1243. doi: 10.3945/ajcn.114.088880. [DOI] [PubMed] [Google Scholar]
- 37.Masuda D., Miyata Y., Matsui S., Yamashita S. Omega-3 fatty acid ethyl esters improve low-density lipoprotein subclasses without increasing low-density lipoprotein-cholesterol levels: A phase 4, randomized study. Atherosclerosis. 2020;292:163–170. doi: 10.1016/j.atherosclerosis.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Gidding S.S., Prospero C., Hossain J., Zappalla F., Balagopal P., Falkner B., Kwiterovich P. A double-blind randomized trial of fish oil to lower triglycerides and improve cardiometabolic risk in adolescents. J. Pediatr. 2014;165:497–503. doi: 10.1016/j.jpeds.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves Luzia L., Mendes Aldrighi J., Teixeira Damasceno N.R., Sampaio G.R., Aparecida R., Soares R.A.M., Silva I.T., De Queiroz Mello A.P., Carioca A.A.F., da Silva Torres A.F. Fish Oil and Vitamin E Change Lipid Profiles and Anti-Ldl-Antibodies in Two Different Ethnic Groups of Women Transitioning through Menopause. Nutr. Hosp. 2015;32:165–174. doi: 10.3305/nh.2015.32.1.9079. [DOI] [PubMed] [Google Scholar]
- 40.Allaire J., Vors C., Harris W.S., Jackson K.H., Tchernof A., Couture P., Lamarche B. Comparing the serum TAG response to high-dose supplementation of either DHA or EPA among individuals with increased cardiovascular risk: The ComparED study. Br. J. Nutr. 2019;121:1223–1234. doi: 10.1017/S0007114519000552. [DOI] [PubMed] [Google Scholar]
- 41.Tani S., Matsuo R., Yagi T., Matsumoto N. Administration of eicosapentaenoic acid may alter high-density lipoprotein heterogeneity in statin-treated patients with stable coronary artery disease: A 6-month randomized trial. J. Cardiol. 2020;75:282–288. doi: 10.1016/j.jjcc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Yokote K., Niwa K., Hakoda T., Oh F., Kajimoto Y., Fukui T., Kim H., Noda Y., Lundström T., Yajima T. Short-Term Efficacy (at 12 Weeks) and Long-Term Safety (up to 52 Weeks) of Omega-3 Free Fatty Acids (AZD0585) for the Treatment of Japanese Patients With Dyslipidemia―A Randomized, Double-Blind, Placebo-Controlled, Phase III Study. Circ. J. 2020;84:994–1003. doi: 10.1253/circj.CJ-19-0358. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Q., Zhang Z., Wang P., Zhang B., Chen C., Zhang C., Su Y. EPA+DHA, but not ALA, Improved Lipids and Inflammation Status in Hypercholesterolemic Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2019;63:e1801157. doi: 10.1002/mnfr.201801157. [DOI] [PubMed] [Google Scholar]
- 44.Rao A., Briskey D., Nalley J.O., Ganuza E. Omega-3 Eicosapentaenoic Acid (EPA) Rich Extract from the Microalga Nannochloropsis Decreases Cholesterol in Healthy Individuals: A Double-Blind, Randomized, Placebo-Controlled, Three-Month Supplementation Study. Nutrients. 2020;12:1869. doi: 10.3390/nu12061869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sunkara A., Raizner A. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. Methodist Debakey Cardiovasc. J. 2019;15:179–184. doi: 10.14797/mdcj-15-3-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manson J.E., Bassuk S.S., Cook N.R., Lee I.M., Mora S., Albert C.M., Buring J.E. VITAL Research Group. Vitamin D, Marine n-3 Fatty Acids, and Primary Prevention of Cardiovascular Disease: Current Evidence. Circ. Res. 2020;126:112–128. doi: 10.1161/CIRCRESAHA.119.314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sable P.S., Kale A.A., Joshi S.R. Prenatal omega 3 fatty acid supplementation to a micronutrient imbalanced diet protects brain neurotrophins in both the cortex and hippocampus in the adult rat offspring. Metabolism. 2013;62:1607–1622. doi: 10.1016/j.metabol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Dhobale M., Joshi S. Altered maternal micronutrients (folic acid, vitamin B 12) and omega 3 fatty acids through oxidative stress may reduce neurotrophic factors in preterm pregnancy. J. Matern. Fetal Neonatal Med. 2012;25:317–323. doi: 10.3109/14767058.2011.579209. [DOI] [PubMed] [Google Scholar]
- 49.Bergami R., Maranesi M., Marchetti M., Sangiorgi Z., Tolomelli B. Influence of dietary n-3 polyunsaturated fatty acids on plasma lipemic effect of vitamin B6 deficiency. Int. J. Vitam. Nutr. Res. 1999;69:315–321. doi: 10.1024/0300-9831.69.5.315. [DOI] [PubMed] [Google Scholar]
- 50.Durand P., Prost M., Blache D. Pro-thrombotic effects of a folic acid deficient diet in rat platelets and macrophages related to elevated homocysteine and decreased n-3 polyunsaturated fatty acids. Atherosclerosis. 1996;121:231–243. doi: 10.1016/0021-9150(95)06724-8. [DOI] [PubMed] [Google Scholar]
- 51.Starkebaum G., Harlan J.M. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J. Clin. Investig. 1986;77:1370–1376. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayden M.S., Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 2014;26:253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones M.L., Mark P.J., Mori T.A., Keelan J.A., Waddell B.J. Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol. Reprod. 2013;88:37. doi: 10.1095/biolreprod.112.103754. [DOI] [PubMed] [Google Scholar]
- 54.Loscalzo J. Homocysteine trials--clear outcomes for complex reasons. N. Engl. J. Med. 2006;354:1629–1632. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 55.Steffen L.M., Folsom A.R., Cushman M., Jacobs D.R., Jr., Rosamond W.D. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: The Longitudinal Investigation of Thromboembolism Etiology. Circulation. 2007;115:188–195. doi: 10.1161/CIRCULATIONAHA.106.641688. [DOI] [PubMed] [Google Scholar]
- 56.Shen J., Wilmot K.A., Ghasemzadeh N., Molloy D.L., Burkman G., Mekonnen G., Gongora M.C., Quyyumi A.A., Sperling L.S. Mediterranean Dietary Patterns and Cardiovascular Health. Annu. Rev. Nutr. 2015;35:425–449. doi: 10.1146/annurev-nutr-011215-025104. [DOI] [PubMed] [Google Scholar]
- 57.Rosato V., Temple N.J., La Vecchia C., Castellan G., Tavani A., Guercio V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019;58:173–191. doi: 10.1007/s00394-017-1582-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhao M., Wu G., Li Y., Wang X., Hou F.F., Xu X., Qin X., Cai Y. Meta-analysis of folic acid efficacy trials in stroke prevention: Insight into effect modifiers. Neurology. 2017;88:1830–1838. doi: 10.1212/WNL.0000000000003909. [DOI] [PubMed] [Google Scholar]
- 59.Chilton F.H., Dutta R., Reynolds L.M., Sergeant S., Mathias R.A., Seeds M.C. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients. 2017;9:1165. doi: 10.3390/nu9111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.