FIGURE 8.
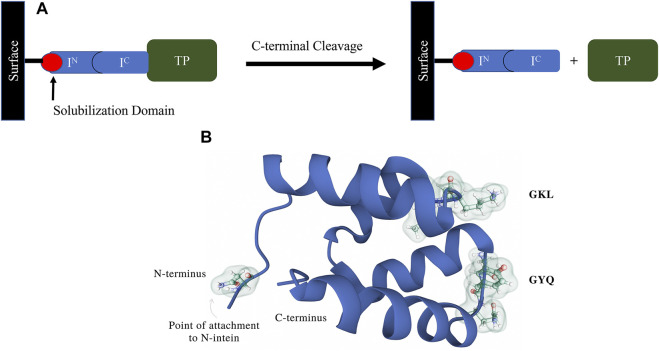
(A). This figure from a patent filed by scientists at Merck KGaA (Zillmann and Orlando, 2019; Patent No.: US 10308679 B2; prioritized in 2014, filed in 2015 and approved in 2019) presents a schematic diagram depicting an affinity purification method based on a self-cleaving tag, as well as a solubilization domain used for manufacturing the split intein ligand. The method employs an affinity chromatography matrix listed in the invention comprising a fusion protein having an N-intein polypeptide fused to an N-intein solubilization partner that is attached to a solid support. A second fusion protein comprising a C-intein that is complementary to the N-intein on the affinity matrix is fused to the target protein to be purified (POI) alongside any other elements required for expression, such as secretion signals. (B). The structure of solubilization partner 138 (p138, PDB ID 1RYK) is also shown (Yee et al., 2002). The protein contains four alpha helix domains, is globular and has a long unstructured coil that forms the connection to the carboxy terminus of the N-intein. The loop regions GKL and GYQ were targeted for cysteine residue insertions away from the catalytic site to create the new versions of p138 that would be functionalized for single-point resin attachment via thiol-coupling chemistry.