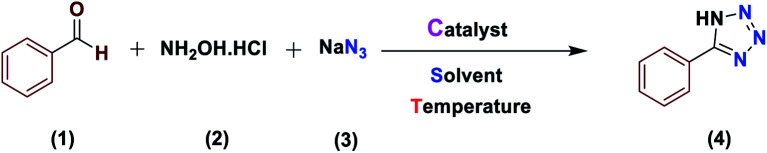
Optimization of the reaction conditions for the synthesis of 5-phenyl-1H-tetrazole.
| |||||
|---|---|---|---|---|---|
| Entry | Solvent | Catalyst (mol%) | Temp. (°C) | Time (min) | Yielda (%) |
| 1 | Water | 0.4 | 80 | 15 | 98 |
| 2 | EtOH | 0.4 | Reflux | 60 | 40 |
| 3 | CH3CN | 0.4 | Reflux | 30 | 55 |
| 4 | DMF | 0.4 | 80 | 30 | 80 |
| 5 | Neat | 0.4 | 80 | 120 | 20 |
| 6 | Water | No catalyst | 80 | 240 | 0 |
| 7 | Water | 0.2 | 80 | 45 | 70 |
| 8 | Water | 0.6 | 80 | 15 | 98 |
| 9 | Water | —b | 80 | 180 | Trace |
| 10 | Water | —c | 80 | 180 | 35 |
| 11 | Water | —d | 80 | 180 | 55 |
| 12 | Water | —e | 80 | 60 | 75 |
| 13 | Water | 0.4 | R.T. | 90 | 10 |
| 14 | Water | 0.4 | 40 | 60 | 60 |
| 15 | Water | 0.4 | 50 | 45 | 82 |
| 16 | Water | 0.4 | 60 | 15 | 97 |
| 17 | Water | 0.4 | 70 | 15 | 98 |
| 18 | Water | 0.4 | 90 | 15 | 95 |
Reaction conditions: aldehyde (1.0 mmol), hydroxylamine (1.2 mmol), and sodium azide (1.5 mmol). The catalyst was prepared using 0.4 mol% catalyst, as explained in the experimental section.
Reaction was performed in the presence of Fe3O4 as the catalyst.
Reaction was performed in the presence of Fe3O4@NFC as the catalyst.
Reaction was performed in the presence of Cu(OAc)2 as the catalyst.
Reaction was performed in the presence of [(5-Cl-Saloph)Cu(ii)]CO2H as the catalyst.
