The synthesis of 5-substituted-1H-tetrazoles catalyzed by (Fe3O4@NFC@NSalophCu)CO2Ha.
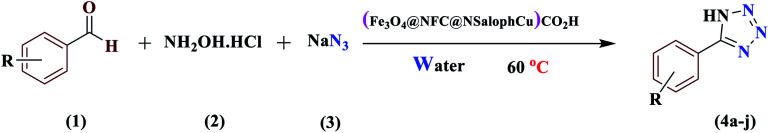
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Aldehyde | Time (min) | Tetrazole | Yieldb (%) | Mp (°C) | TON | TOF | Ref. (lit) |
| 1a |
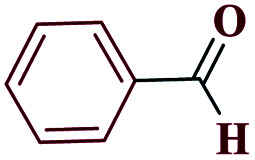
|
15 |
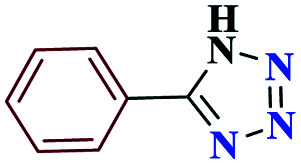
|
97 | 217–218 | 242.5 | 970 | 60 |
| 2b |
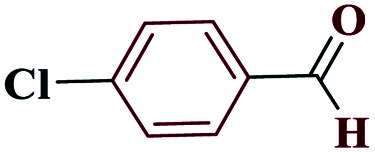
|
15 |
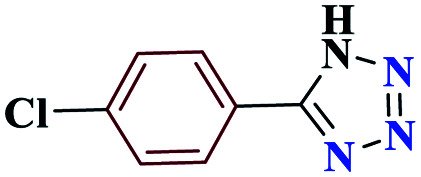
|
97 | 258–259 | 242.5 | 970 | 60 |
| 3c |
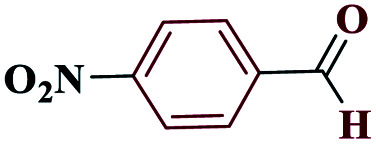
|
15 |
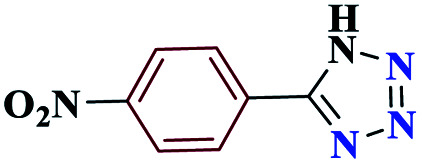
|
97 | 220–221 | 242.5 | 970 | 60 |
| 4d |
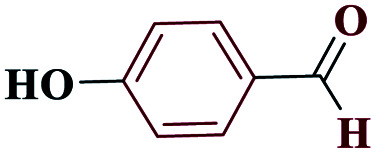
|
20 |
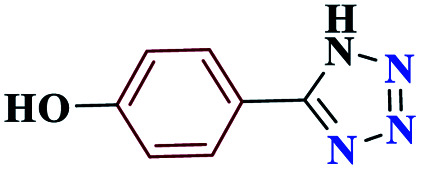
|
90 | 233–234 | 225 | 682 | 60 |
| 5e |
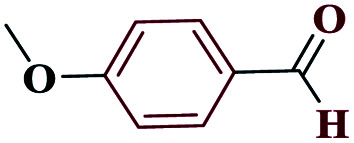
|
10 |
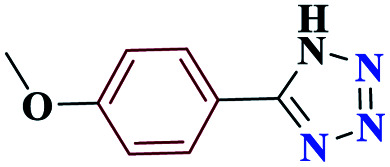
|
98 | 232–233 | 245 | 1476 | 60 |
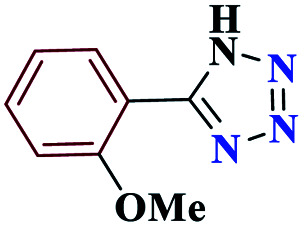
| 6f |
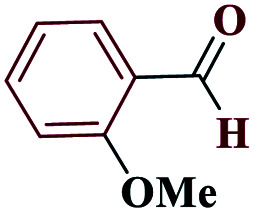
|
15 |
|
98 | 154–156 | 245 | 980 | 60 |
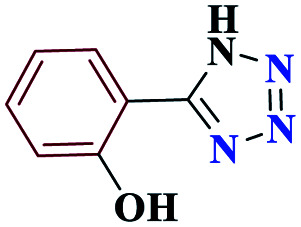
| 7g |
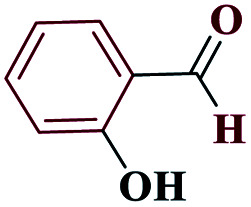
|
30 |
|
85 | 220–221 | 212.5 | 425 | 60 |
| 8h |
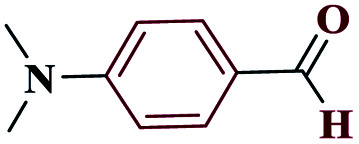
|
10 |
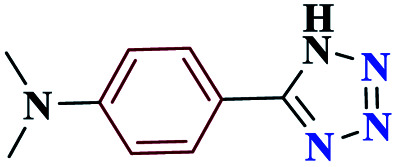
|
98 | 133–134 | 245 | 1476 | 60 |
| 9i |
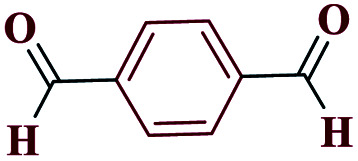
|
15 |
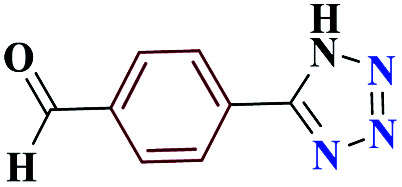
|
92 | 187–189 | 230 | 920 | 53 |
| 10j |
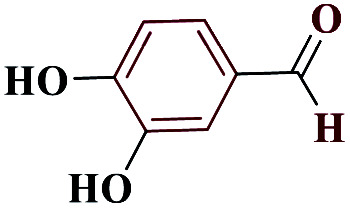
|
10 |
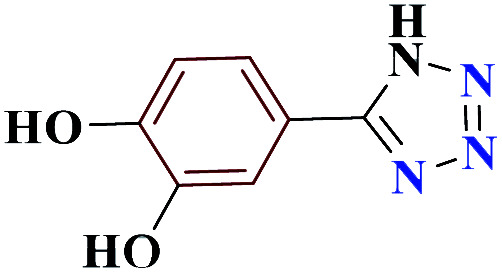
|
75 | 214–215 | 187.5 | 1130 | 60 |
Reaction conditions: aldehyde (1.0 mmol), hydroxylamine (1.2 mmol), sodium azide (1.5 mmol), catalyst (0.4 mol%), and H2O (5 mL); 60 °C.
Isolated yield.
