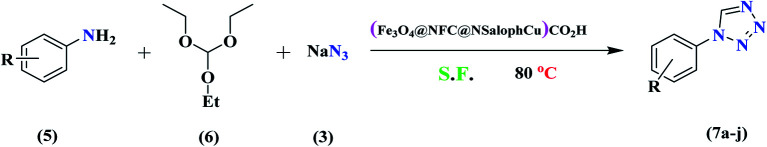
The synthesis of 1-substituted-1H-tetrazoles catalyzed by (Fe3O4@NFC@NSalophCu)CO2Ha.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Aldehyde | Time (min) | Tetrazole | Yieldb (%) | Mp (°C) | TON | TOF | Ref. (lit) |
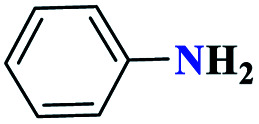
| 1a |
|
20 |
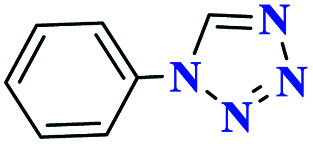
|
98 | 63 | 326.6 | 990 | 61 |
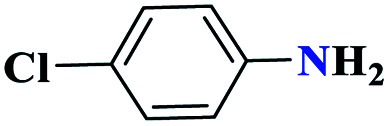
| 2b |
|
30 |
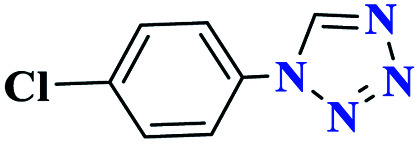
|
90 | 155 | 300 | 600 | 61 |
| 3c |
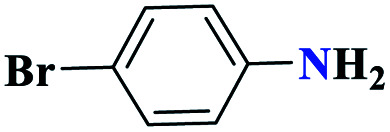
|
30 |
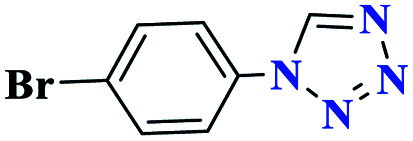
|
95 | 176–177 | 316.6 | 633 | 61 |
| 4d |
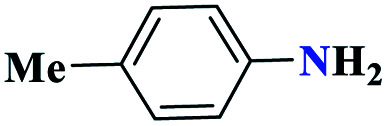
|
15 |
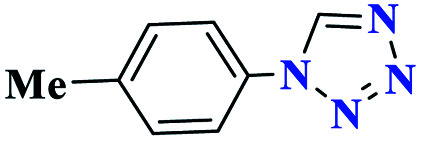
|
90 | 92–93 | 300 | 682 | 61 |
| 5e |
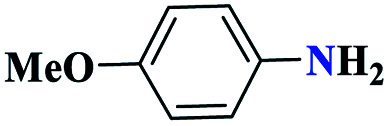
|
15 |
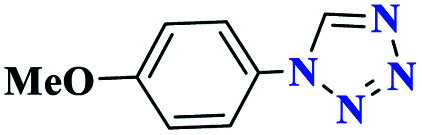
|
97 | 114–115 | 323.3 | 1293 | 61 |
| 6f |
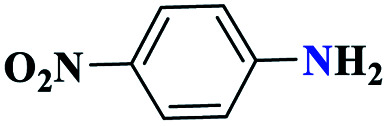
|
45 |
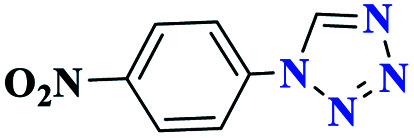
|
80 | 200 | 266.6 | 355.4 | 61 |
| 7g |
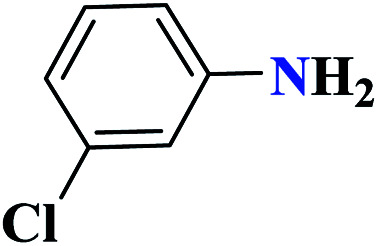
|
50 |
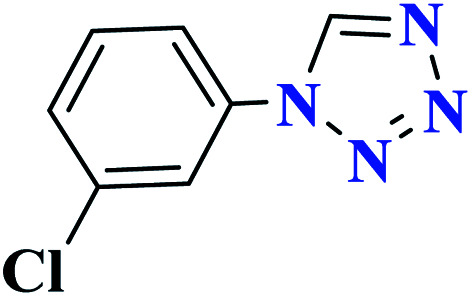
|
90 | 139–140 | 300 | 360 | 62 |
| 8h |
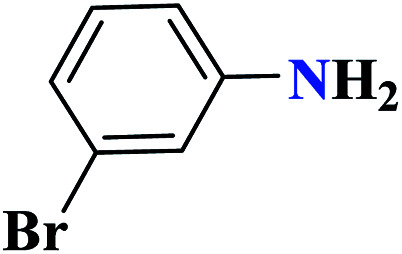
|
60 |
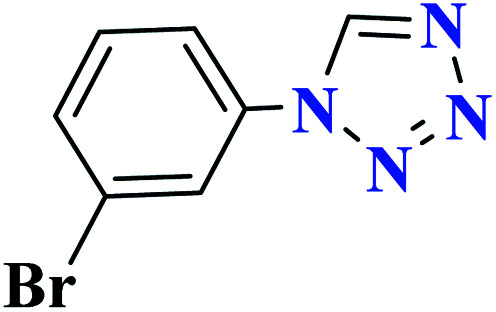
|
90 | 78–79 | 300 | 300 | 1 |
| 9i |
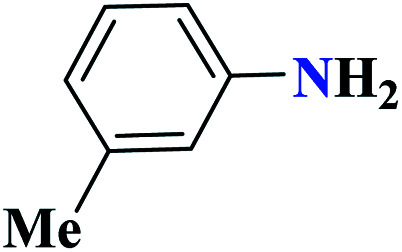
|
30 |
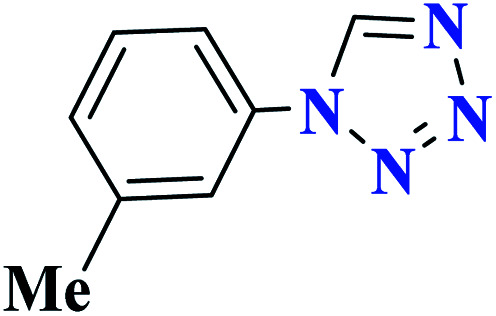
|
97 | 55 | 323.3 | 646.5 | 63 |
| 10j |
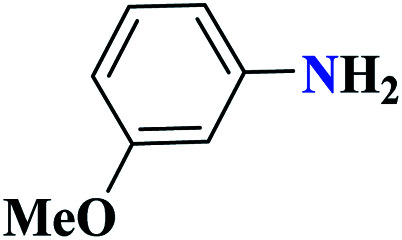
|
30 |
|
97 | 67–68 | 323.3 | 646.5 | 64 |
Reaction conditions: aniline (1.0 mmol), triethyl orthoformate (1.2 mmol), sodium azide (1.2 mmol), catalyst (0.3 mol%), and solvent-free conditions; 80 °C.
Isolated yield.
