A list of supercharging reagents and some of their physico-chemical propertiesa.
| Common name/[IUPAC name]α | Molecular structureα | Ave. mass,α Da | Boiling ptα °C at 760 mmHg | Vapour press.αβ mmHg at 25 °C | Surface tensionαβ mN m−1 at 25 °C | Densityαβ (specific gravity) 25 °C | Acidity pKa 25 °C | Dipole momentδ μ(D)/μD* | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3- to 4-membered & planar molecules | ||||||||||
| 1 | Dimethyl sulfoxide (DMSO) [(Methylsulfinyl)methane] |
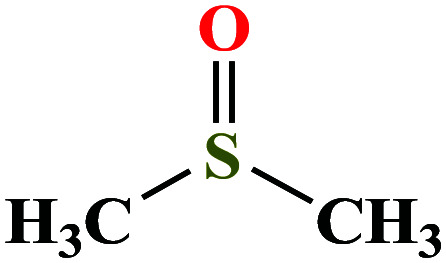
|
78.133 | 189 | 0.60 | 43.54 | 1.101 (ref. 65) | 35.1 | 3.96/4.44 | 3 and 25 |
| 2 | Ethylene glycol [1,2-ethandiol] |

|
62.068 | 196–198 | 0.06 | 47.99 | 1.113 | 14.22 | 2.747/— | 3 |
| 3 | Glycerol [1,2,3-propanetriol] |
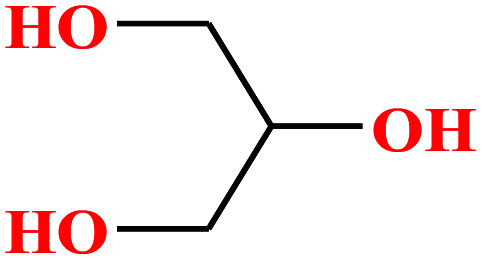
|
92.094 | 182 | 1.68 × 10−4 | 72.6 | 1.26 (ref. 65) | 14.4 | 2.68/— | 5 and 8 |
| 4 | Formamide |
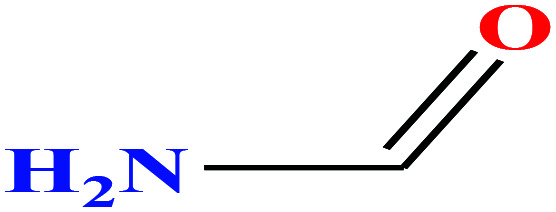
|
45.041 | 210 | 6.10 × 10−2 (ref. 66) | 58.35 (ref. 67) | 1.13 | 23.5 (in DMSO) | — | 3 |
| 5 | 2-Methoxyethanol |
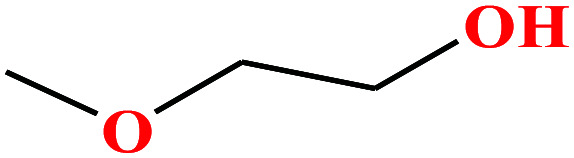
|
76.094 | 124–125 | 6.2 (ref. 68) | 30.84 (ref. 69) | 0.97 (ref. 65 and 70) | 14.8 | — | 3 |
| 6ε | Methoxypropanol [1-methoxypropan-2-ol] |
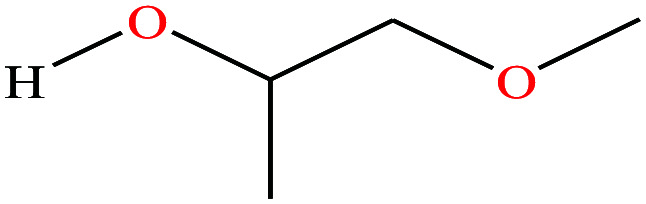
|
90.122 | 120 | 10.9 | 2.64 × 10−3 | 0.926 | 14.49 ± 0.20 (ref. 70 and 71) | — | |
| 7 | Crotononitrile[(2E)-2-butenenitrile] |

|
67.089 | 120–121 | 31.95 | — | — | — | 4.3/— | 15 |
| Heterocyclic 4- & 5-membered ring structures, including organosulfur cpd (sulfones) | ||||||||||
| 8 | Dimethyl sulfone [methylsulfonylmethane] |
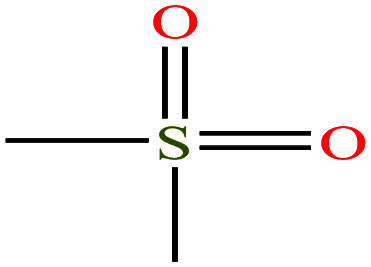
|
94.133 | 238 | — | — | 1.45 | 31 | 4.5/— | 15 |
| 9 | N,N,N′N′-Tetraethylsulfamide (TES) [N-(diethylsulfamoyl)-N-ethylethanamine] |
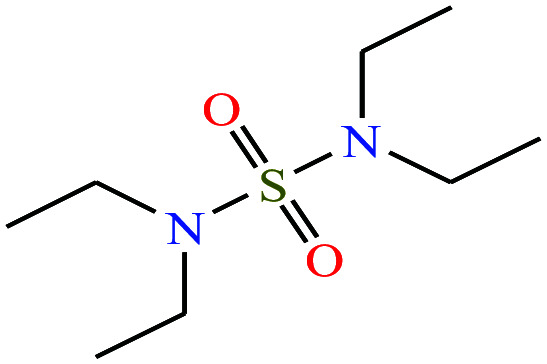
|
208.32 | 125 | — | — | 1.283 | — | — | 16 |
| 10 | 3-Chlorothiete-1,1-dioxide |
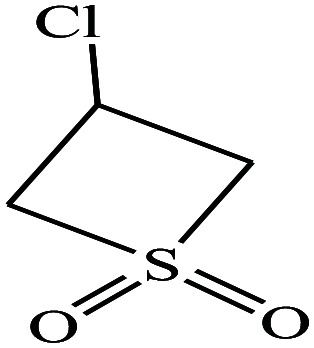
|
140.59 | 328.9 | — | — | 1.64 | — | —/3.38 | 9 |
| 11ε | 3-Chloro-2H-thiete 1,1-dioxide |
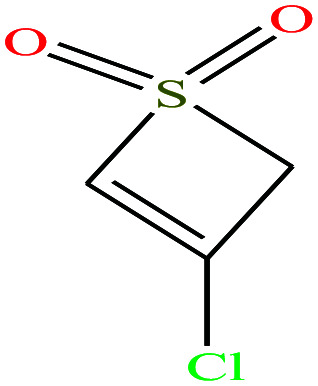
|
138.573 | 328.9 ± 41.0 | 3.2 ± 0.3 | — | 1.6 ± 0.1 | — | — | |
| 12 | 2-Thiophenone [2H-thiophen-5-one] |
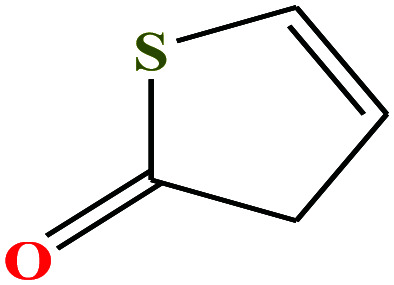
|
100.139 | 197.4 | 0.4 ± 0.3 | 38.7 ± 3.0 | 1.24 | 49 | ||
| 13ε | 4-Butyrothiolactone [thiolan-2-one] |
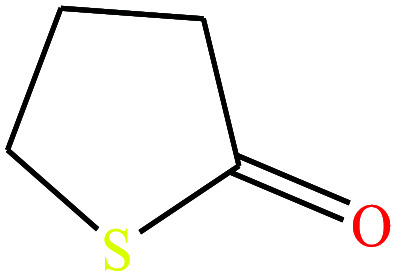
|
102.16 | 76.5429 | — | — | 1.18 | Poorly soluble in water | — | |
| 14 | Sulfolane [2,3,4,5-tetrahydrothiophene-1,1-dioxide] |
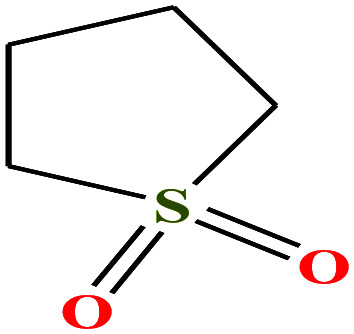
|
120.170 | 285 | 6.2 × 10−3 (27.6 °C) | 35.5 (ref. 72) | 1.26 (ref. 65) | 12.9 | 4.35/5.68 | 7, 8, 52 and 53 |
| 15 | Sulfolene [2,5-dihydrothiophene 1,1-dioxide] |
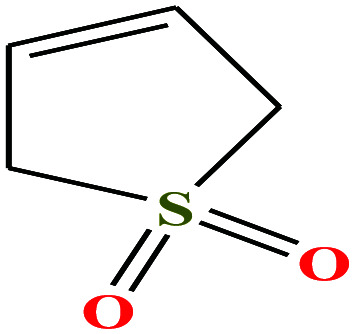
|
118.154 | 64–65.5 °C mpt (ref. 73) | — | 41.0 | 1.3 (ref. 68) | — | —/5.69 | 47 |
| 16 | 1,3-Propanesultone [1,2-oxathiolane 2,2-dioxide] |
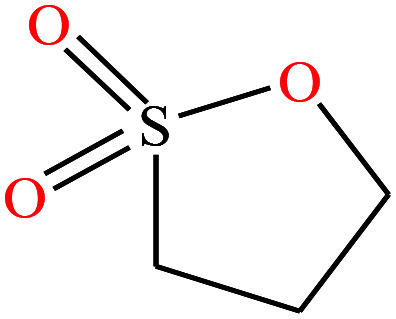
|
122.143 | 315.87 | — | — | 1.392 | — | — | 15 |
| 17 | 1,4-Butanesultone [1,2-oxathiane 2,2-dioxide] |
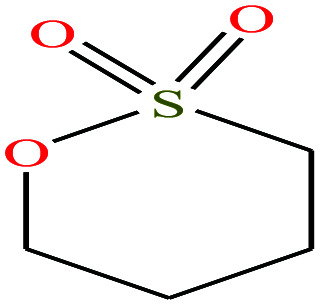
|
136.169 | 304.047 | — | — | 1.335 | — | — | 15 |
| 18 | 3-Methyl-2-oxazolidone (MOZ) [3-methyl-1,3-oxazolidin-2-one] |
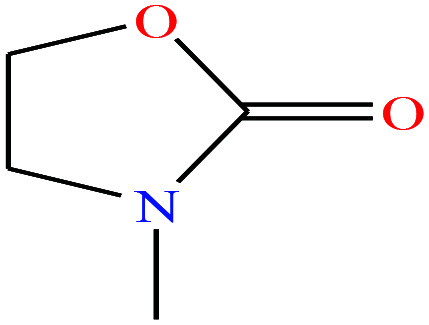
|
101.1 | 88 | — | — | 1.17 | — | — | 16 |
| Heterocyclic 4- & 5-membered ring structures (including the heterocyclic acetals) | ||||||||||
| 19 | Glycerol carbonate [4-hydroxymethyl-1,3-dioxolan-2-one] |
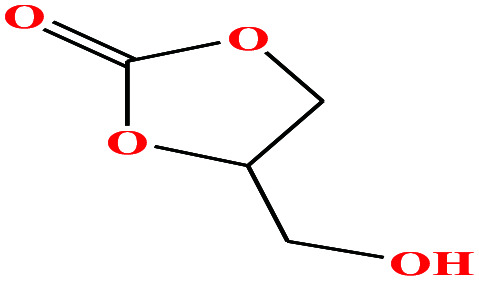
|
118.088 | 353.9 ± 15 | 0.0 ± 1.8 | 41.1 | 1.375 | — | — | 48 |
| 20 | Propylene carbonate [4-methyl-1,3-dioxol-2-one] |
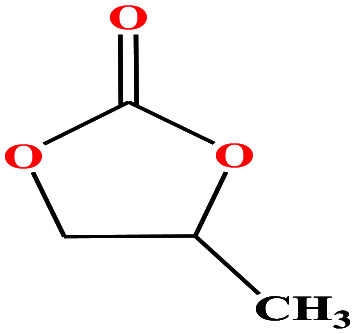
|
102.089 | 240 | 0.045 (ref. 66) | 45 | 1.2 (ref. 69) | — | 4.9 | 48 and 49 |
| 21 | Ethylene carbonate [1,3-dioxolan-2-one] |
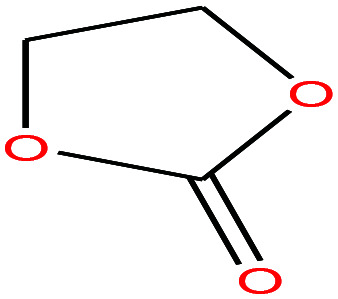
|
88.062 | 244–245 | — | 54 (30 °C) | 1.32 | — | 4.9/— | 15 |
| 22 | Butylene carbonate [4-ethyl-1,3-dioxolan-2-one] |
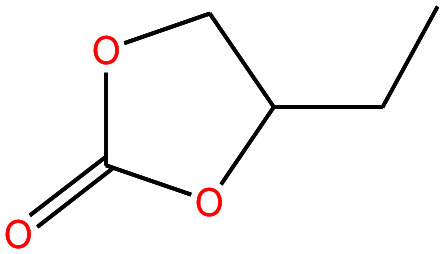
|
116.115 | 281.18 | — | — | 1.141 | — | — | 15 |
| 23 | Vinylethylene carbonate [4-vinyl-1,3-dioxolan-2-one] |
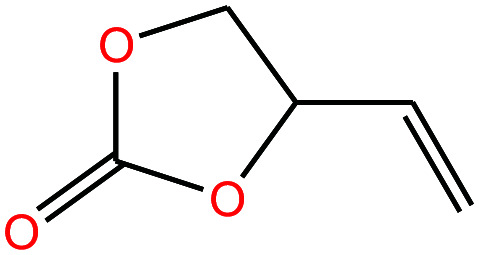
|
114.099 | 238.72 | — | — | 1.188 | — | — | 15 |
| Benzene-ring containing molecules | ||||||||||
| 24 | Nitrobenzene |
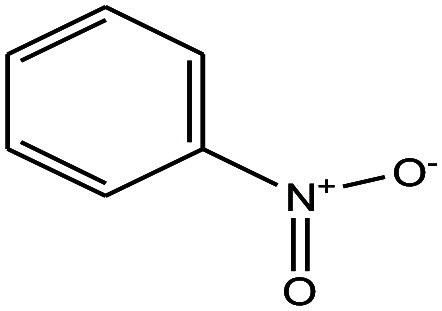
|
123.109 | 210–211 | 4.2 | 0.245 | 1.201 | 1.204 slightly soluble in water | 4.2/— | 15 |
| 25 | Benzyl alcohol [(hydroxymethyl)benzene] |
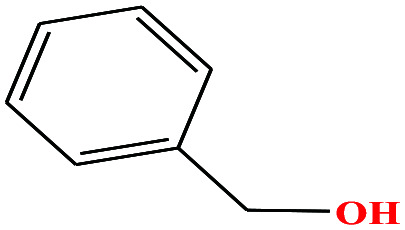
|
108.138 | 205 | 0.094 (ref. 66) | 39.0 (ref. 70) | 1.0 (ref. 65) | 15.4 | 1.71/1.79 | 8 |
| 26 | m-Chlorophenol [3-chlorophenol] |
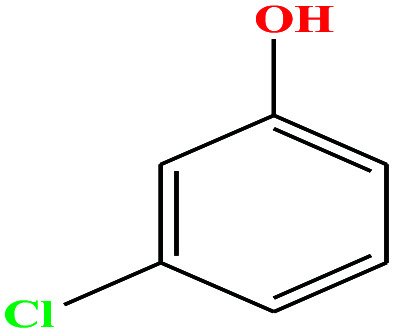
|
128.556 | 214 | 0.125 | 44.7 | 1.3 (ref. 68) | 9.12 | 1.03 ± 1.08 | 4 |
| 27ε | o-Chlorophenol [2-chlorophenol] |
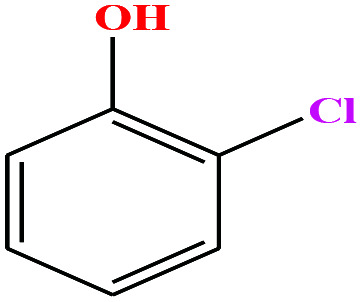
|
128.556 | 175–176 | 2.53 (ref. 66) | 40.50 | 1.26 (ref. 65) | 8.56 | — | |
| 28ε | p-Chlorophenol [4-chlorophenol] |
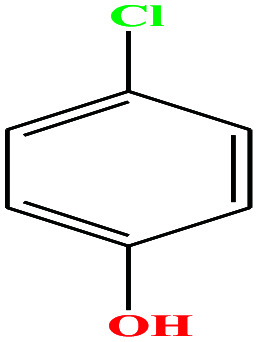
|
128.556 | 217–220 | 1 (ref. 74) | 8.7 × 10−2 mm Hg | 1.306 (ref. 65) | 9.41 | 2.10/— | |
| 29 | 2-Nitrochlorobenzene [1-chloro-2-nitrobenzene] |
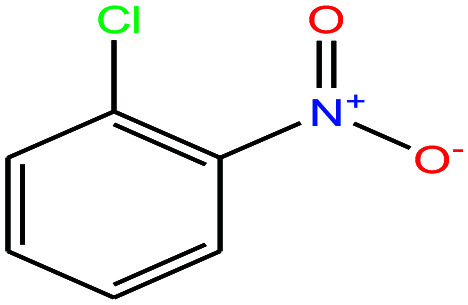
|
157.555 | 245–246 | — | 48.4 ± 3.0 | 1.348 | 0.6 | 4.6/— | 15 |
| 30ε | 3-Nitrochlorobenzene [1-chloro-3-nitrobenzene] |
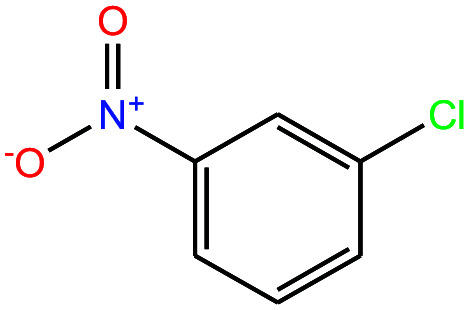
|
157.555 | 235–236 | 0.097 (ref. 66) | 4.37 × 10−2 | 1.3 | — | — | |
| 31ε | 4-Nitrochlorobenzene [1-chloro-4-nitrobenzene] |
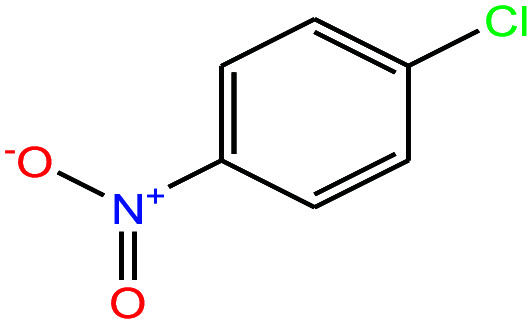
|
157.555 | 241.66 | 2.19 × 10−2 (ref. 66) | 3.71 × 10−2 | 1.520 | n/a | — | |
| 32 | o-Nitrobenzyl alcohol (oNBA) [2-nitro(phenyl)methanol] |
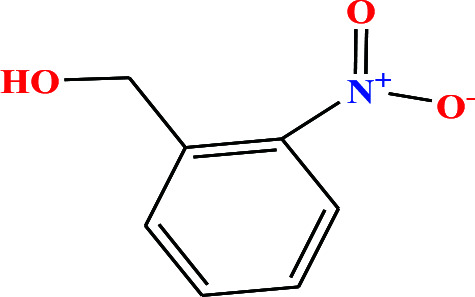
|
153.135 | 270 (solid at RT) | 0.0 ± 0.5 | — | 1.3 | — | — | 8 |
| 33 | m-Nitrobenzyl alcohol (mNBA) [3-nitro(phenyl) methanol] |
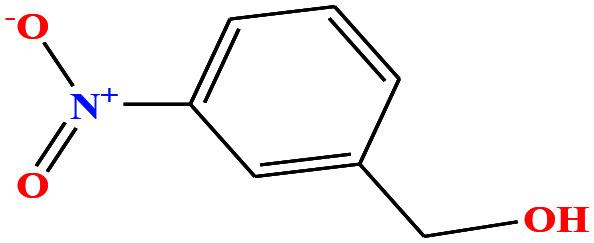
|
153.135 | 349.8 | 0.0 ± 0.5 | 50 ± 5 | 1.29 | — | — | 4, 6, 7, 8, 48, 49 and 75 |
| 34 | p-Nitrobenzyl alcohol (pNBA) [4-nitro(phenyl)methanol] |
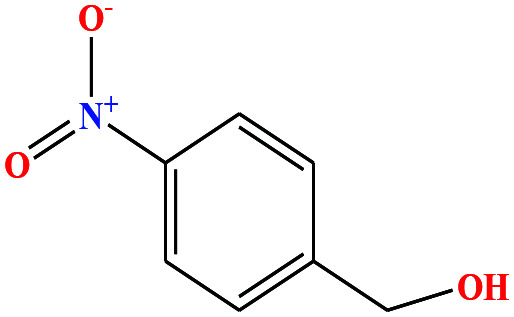
|
153.135 | 185 (solid at RT) | Negligible | — | — | 7.15 | — | 8 |
| 35 | m-Nitroacetophenone [1-(3-nitrophenyl)ethanone] |
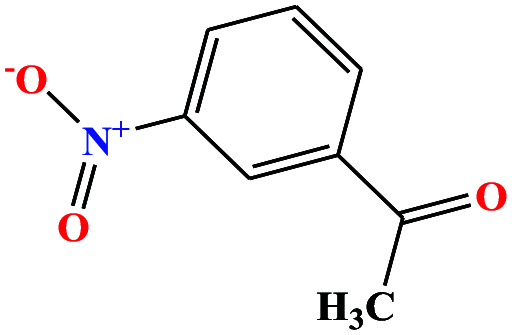
|
165.146 | 202 (solid at RT) | 3.86 × 10−5 | — | 1.4 | — | — | 8 and 27 |
| 36 | m-Nitrobenzonitrile [3-benzonitronitrile] |
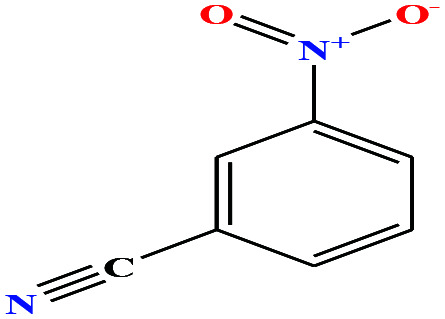
|
148.119 | 165 | 0.0 ± 0.5 | — | 1.3 ± 0.1 | — | — | 6, 8 and 27 |
| 37ε | o-Nitrobenzonitrile [2-benzonitronitrile] |
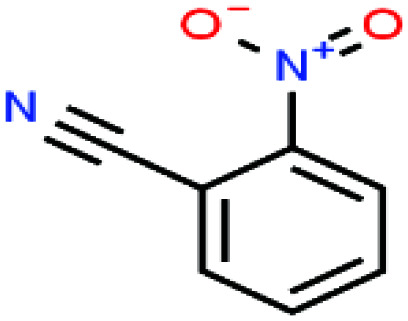
|
148.119 | 321.78 165.0 °C (16.0 mmHg) | Insoluble in water | — | — | — | — | |
| 38ε | p-Nitrobenzonitrile [4-benzonitronitrile] |
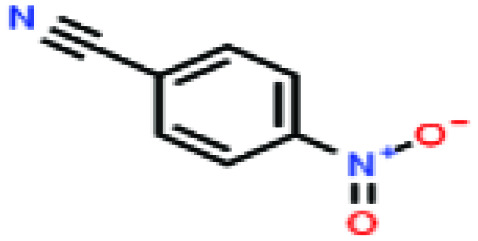
|
148.119 | — | — | — | — | — | — | |
| 39 | m-nitrophenyl ethanol [1-(3-nitrophenyl)ethanol] |
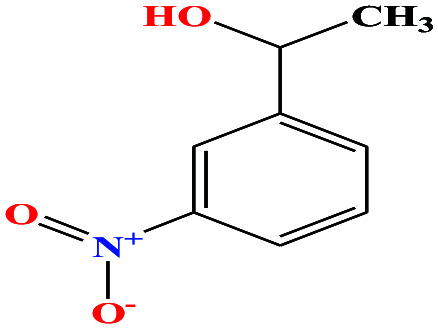
|
167.162 | 281.3 ± 23.0 | 0.0 ± 0.6 | — | 1.3 ± 0.1 | — | — | 6 and 8 |
| 40ε | o-Nitrophenyl ethanol [1-(2-nitrophenyl)ethanol) |
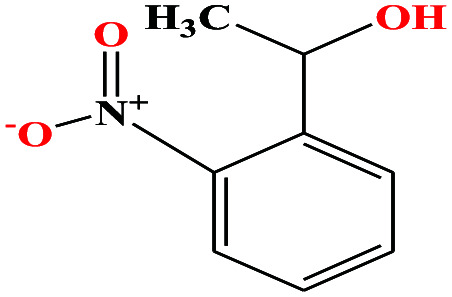
|
167.162 | 319.0 ± 17.0 | 0.0 ± 0.6 | — | 1.3 ± 0.1 | — | — | |
| 41ε | p-Nitrophenyl ethanol (1-(4-nitrophenyl)ethanol] |
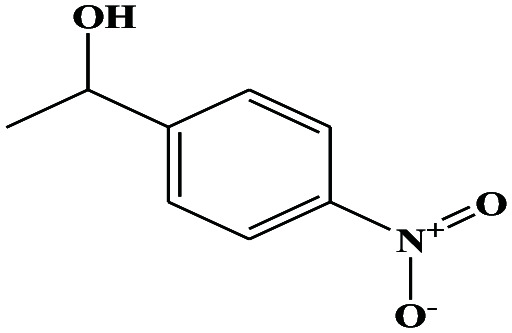
|
167.162 | 290.68 | 0.0 ± 0.7 g | — | 1.3 ± 0.1 | — | — | |
| 42 | o-Nitroanisole [1-methoxy-2-nitrobenzene] |
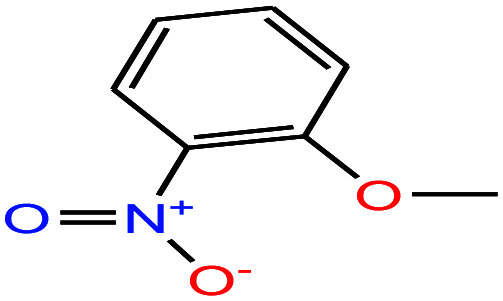
|
153.135 | 272–273 | — | 48 | 1.254 | — | 5.0/— | 15 |
| 43ε | m-Nitroanisole [1-methoxy-3-nitrobenzene] |
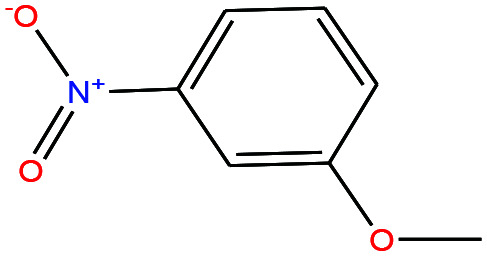
|
153.135 | 256.3 ± 13.0 | 0.0 ± 0.5 | — | 1.2 ± 0.1 | — | — | |
| 44 | p-Nitroanisole [1-methoxy-4-nitrobenzene] |
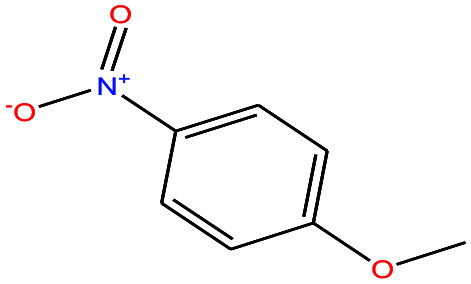
|
153.135 | 260 | — | — | 1.233 | — | 5.3/— | 15 |
| 45 | 3-Nitrophenethyl alcohol [2-(3-nitrophenyl)ethanol] |
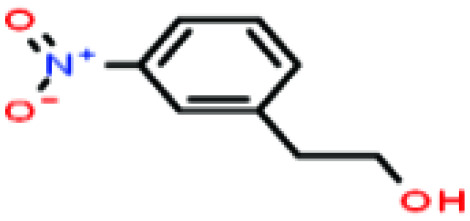
|
167.162 | 341.7–349.0 | — | — | — | — | — | 8 |
| 46ε | 2-Nitrophenethyl alcohol [2-(2-nitrophenyl)ethanol] |
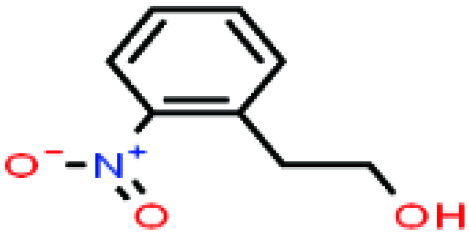
|
167.162 | 267 | — | — | 1.19 | — | — | |
| 47ε | 4-Nitrophenethyl alcohol [2-(4-nitrophenyl)ethanol] |
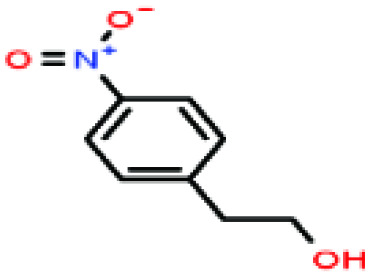
|
167.162 | 337 | — | — | — | — | — | |
| 48 | m-(Trifluoromethyl)-benzyl alcohol [[3-(trifluoromethyl)phenylmethanol] |
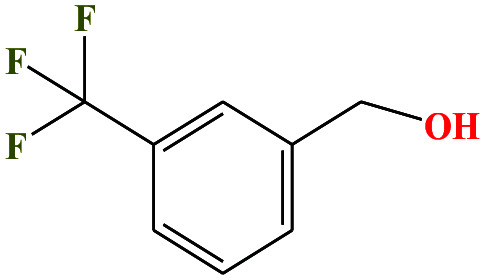
|
176.136 | 257–261 | 0.1 ± 0.4 | — | 1.3 ± 0.1 | — | — | 6 |
| 49ε | o-(Trifluoromethyl)-benzyl alcohol [2-(trifluoromethyl)phenylmethanol] |
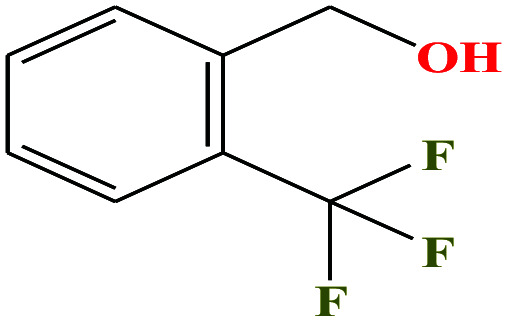
|
176.136 | 214–262 | — | — | 1.3 ± 0.1 | — | — | |
| 50ε | p-(Trifluoromethyl)-benzyl alcohol [4-(trifluoromethyl)benzyl alcohol] |
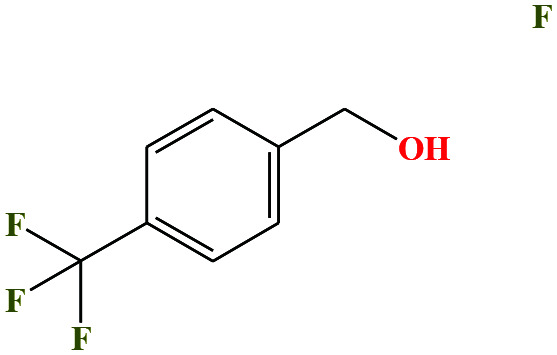
|
176.136 | 250 | 0.1 ± 0.4 | — | 1.28 (ref. 74) | — | — | |
| 51ε | o-Nitrobenzoic acid [2-nitrobenzoic acid] |
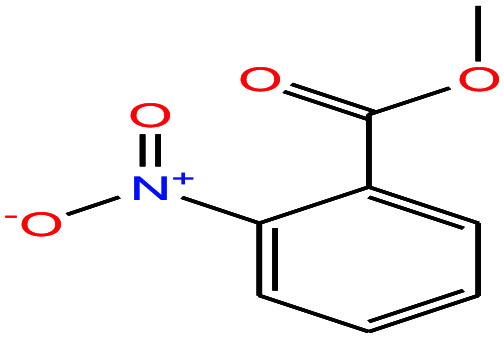
|
167.12 | 295.67 | 0.0 ± 0.8 (ref. 75) | 66.4 ± 3.0 | 1.575 | 2.17 | 4.07/— | |
| 52ε | m-Nitrobenzoic acid [3-nitrobenzoinc acid] |
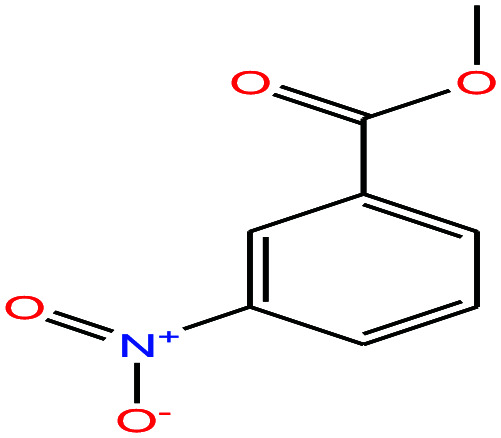
|
167.12 | 340.7 ± 25.0 | 3.71 × 10−5 (ref. 75) | 66.4 ± 3.0 | 1.5 ± 0.1 | 3.47 | 4.03/— | |
| 53ε | p-Nitrobenzoic acid [4-nitrobenzoic acid] |
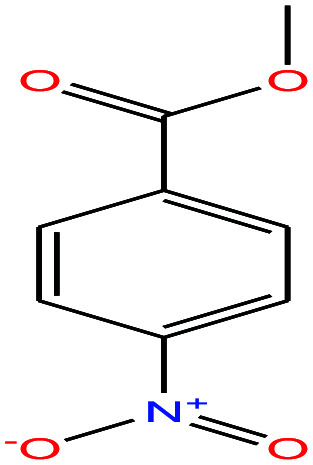
|
167.12 | It sublimes | — | — | 1.58 | 3.41 | 4.05/— | |
| Typical solvents used in ESI | ||||||||||
| Common name/IUPAC name | Molecular structure | Ave. mass, Da | Boiling pt °C at 760 mmHg | Vap. press. mmHg at 25 °C (ref. 80) | Surface tension mN m−1 at 25 °C | Density (specific gravity) | Acididty pKa 25 °C | Dipole moment μ(D)/μD* | 66 and 69 | |
| 1 | Water |
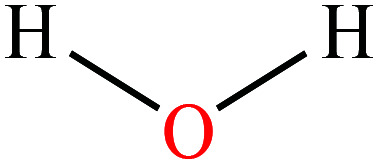
|
18.02 | 100 | 17.44 | 72.8 | 1.00 | 14 | 1.85/2.16 | |
| 2 | Methanol |
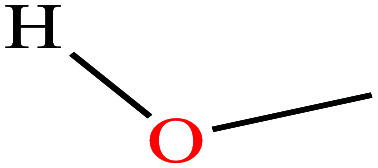
|
32.04 | 64.7 | 127 | 22.07 | 0.792 | 15.5 | 1.70/ | |
| 3 | Ethanol |
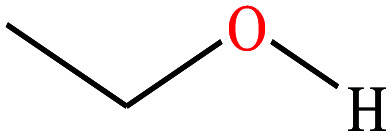
|
46.07 | 78.2 | 59.3 | 21.97 | 0.789 | 15.9 | 1.69/ | |
| 4 | Acetonitrile |
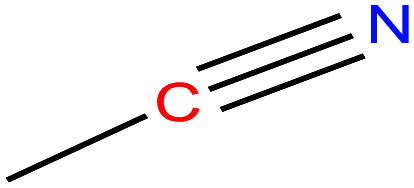
|
41.05 | 81.3–82.1 | 88.8 | 29.04 | 0.786 | 25 | 3.92/ | |
| 5 | Ethyl acetate |
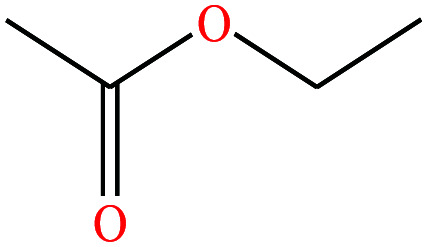
|
88.106 | 77.1 | 93.2 | 24 | 0.902 | 25 | 1.78/ | |
| 6 | Acetone |
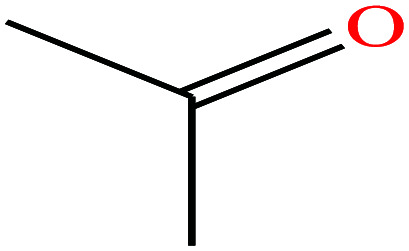
|
58.08 | 56.05 | 231 | 23.7 | 0.785 | 19.16 | 2.91/ | |
Key: α chemspider: http://www.chemspider.com/, γ pKaε candidate isomers proposed supercharging reagents, β pubchem: https://pubchem.ncbi.nlm.nih.gov/, δ dipole moment μ(D)/μD*: determined by experiment/calculated*. We acknowledge that some values in Table 1 are incomplete.
