Figure 7.
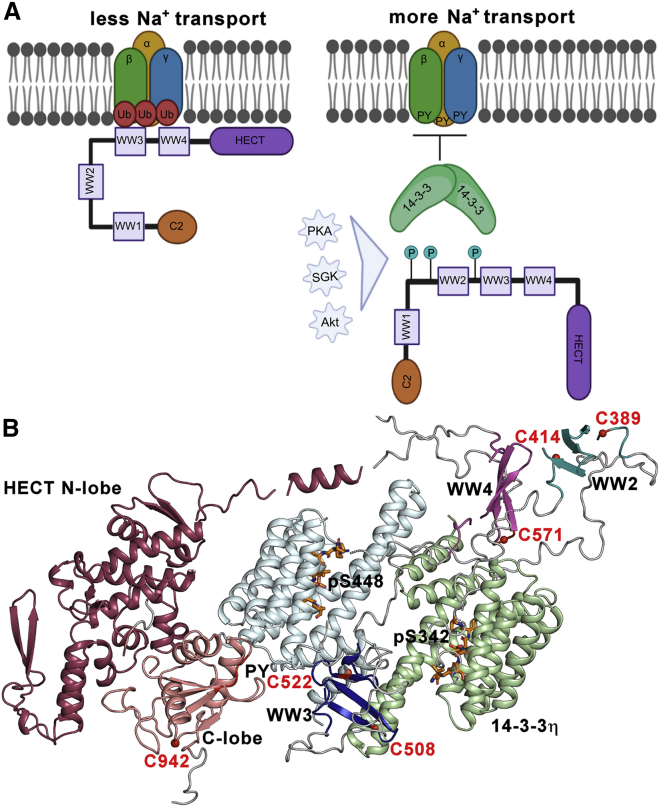
Phosphorylation followed by 14-3-3 binding regulates Nedd4-2. (A) Nedd4-2 binds to ENaC PY motifs and catalyzes its ubiquitination, reducing the rate of sodium transport and consequently the surface expression of these channels. Phosphorylation by various kinases (PKA, SGK, and Akt) triggers 14-3-3 protein binding, which sterically blocks WW domains and affects the structure of the active site, thereby preventing Nedd4-2 binding to ENaC and its ubiquitination. The 14-3-3 binding motifs S342, T367, and S448 are shown as teal circles with P. (B) Positions of AEDANS-labeled cysteine residues in the SAXS-based model of the pNedd4-2186−975:14-3-3η complex (30). The 14-3-3η protomers are shown in pale green and pale cyan. In the HECT domain of Nedd4-2186−975, the N-lobe is indicated in raspberry and the C-lobe in salmon. WW2, WW3, and WW4 domains are indicated in teal, blue, and magenta, respectively. Phosphorylated 14-3-3 binding motifs of Nedd4-2 are shown as orange sticks (PDB: 6ZBT and 6ZC9 (30)). The positions of the cysteine residues in the WW2, WW3, WW4, and HECT domains labeled by AEDANS are indicated as red balls. The WW1 domain is not shown. The PY motif (L948PPY951) is shown in red. To see this figure in color, go online.