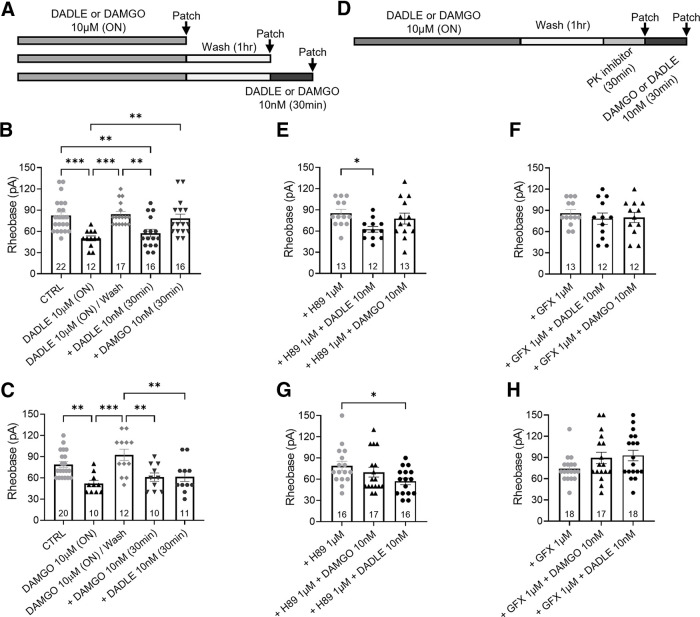
Figure 8.
Overnight exposure to high-concentration opioids switches low-concentration DOPr and MOPr agonist responses to a PKC-dependent pronociceptive pathway. A, Schematic protocol showing the paradigm used in B and C. Arrows indicate perforated patch-clamp recordings at different treatment points. B, The pronociceptive effect induced by high-concentration DADLE is lost after 1 h washout with control media, re-exposure to a low-concentration DADLE paradoxically decreased neuron rheobase (F(4,78) = 8.474, p < 0.0001). C, After washout of high-concentration DAMGO overnight, acute re-exposure to DAMGO or DADLE paradoxically decreased neuron rheobase (F(4,58) = 7.111, p < 0.0001). D, Schematic protocol showing the paradigm used in E–H. Arrows indicate perforated patch-clamp recordings after incubation with PKA or PKC inhibitors with or without opioid re-exposure. E, F, Following the washout of overnight high-concentration DADLE, PKC inhibitor GFX (F: F(2,34) = 0.382, p = 0.686) but not PKA inhibitor H89 (E: F(2,35) = 3.681, p = 0.035) prevented the paradoxical increased excitability induced by acute re-exposure to low-concentration DADLE. G, H, Following washout of overnight high-concentration DAMGO, PKA inhibitor H89 (G: F(2,53) = 4.1, p = 0.022) only prevented the pronociceptive effect induced by acute re-exposure to low-concentration DAMGO while PKC inhibitor GFX (H: F(2,50) = 2.246, p = 0.116) prevented the increased excitability induced by acute re-exposure to both low-concentration DAMGO or DADLE. Number of cells appears in each bar. Error bars indicate mean ± SEM. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; one-way ANOVA with Dunnett's post hoc test.