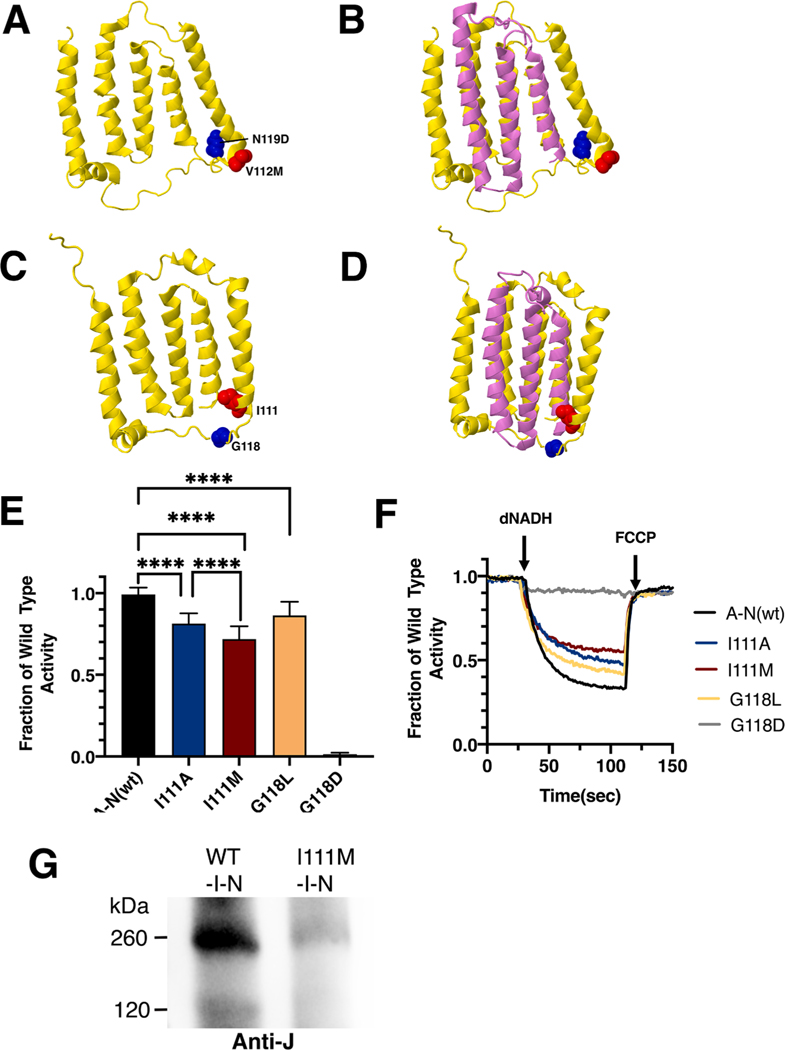
Fig. 9.
Analysis of ND6 mutations in nuoJ of E. coli. (A) The locations of human mutations V112M (red) and N117D (blue) are shown in ND6 (yellow), using PDB file 5xtd (Gu et al., 2016). (B) Neighboring subunit ND4L (violet) is shown. (C) The locations of E. coli residues I111 (red) and G118 (blue) are shown in nuoJ (yellow), using PDB file 3rko (Efremov and Sazanov, 2011). (D) Neighboring subunit nuoK (violet) is shown. (E) dNADH-oxidase activities of membrane vesicles prepared from the E. coli mutants are shown compared to a wild type sample prepared the same day. (F) Proton translocation rates from the same samples shown in panel E are indicated by fluorescence quenching of the acridine dye ACMA. (G) Membrane vesicles were prepared from cells expressing wild type pBAD33-(I-N) and from the same plasmid carrying the I111M mutation. The samples were solubilized with dodecyl maltoside and analyzed by BN gel electrophoresis. After probing with the blot with anti-J antibody, the wild type sample showed the sub-complex of JKLMN (~260 kDa), while the mutant showed only a faint band at that position. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)