Figure 5. CD38 affects cardiomyocytes’ calcium handling by the sarcoplasmic reticulum.
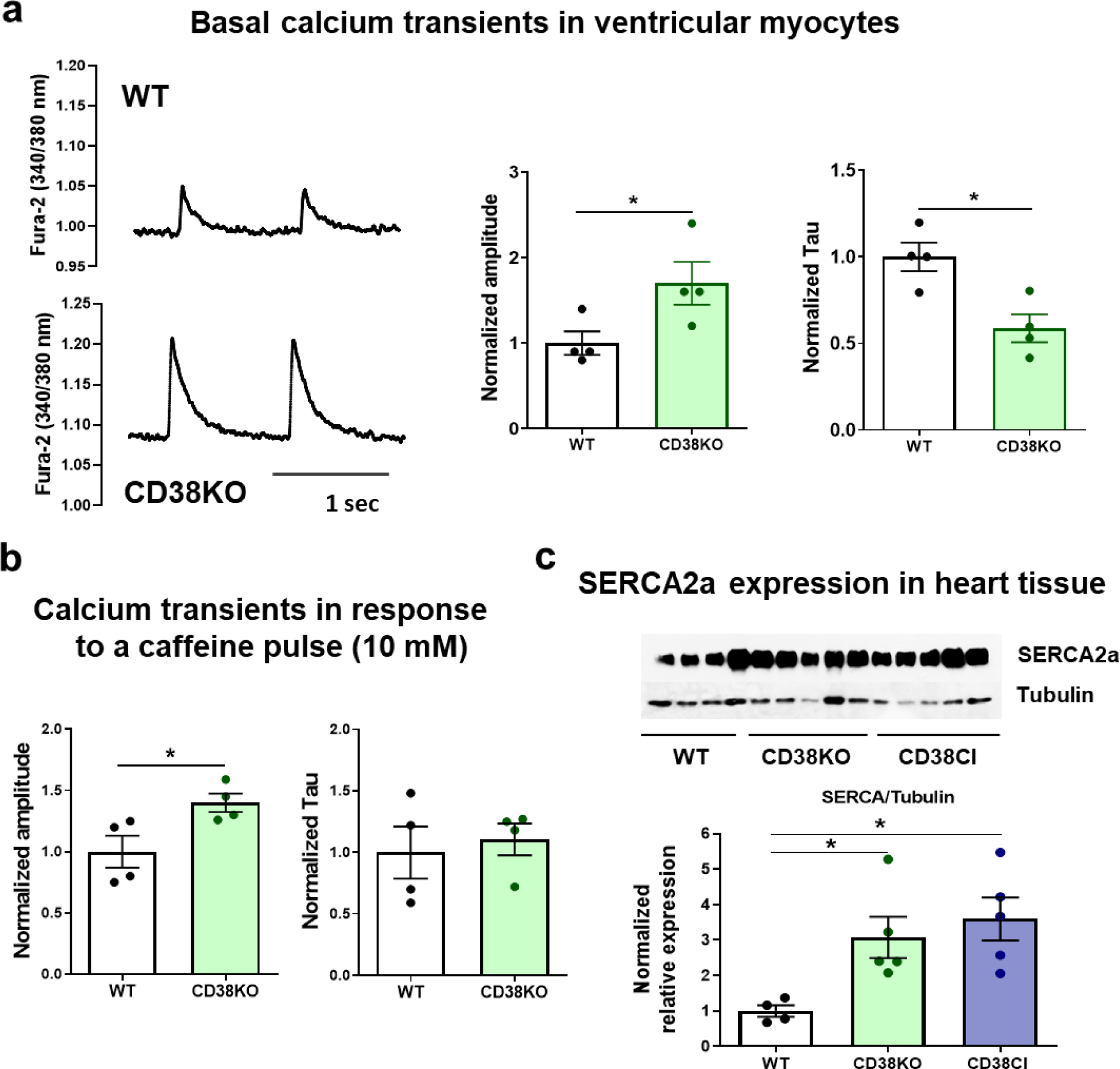
Hearts from WT and CD38KO adult male mice (6–8-month-old) were isolated in order to either obtain cardiomyocytes or lysates for immunoblotting. (a) Representative traces of calcium transients from WT and CD38KO ventricular cardiomyocytes electrically stimulated at 1 Hz at room temperature and its corresponding quantification. Isolated cardiomyocytes from CD38KO mice show increased calcium transient amplitude and faster time decay constant compared to WT cells. Each point in the graphs represents the average value for all the cells recorded for each mice (N=4 mice/group, n=22 and 30 for WT and CD38KO groups respectively). (b) Quantification of calcium transients in response to a caffeine pulse (10 mM). Isolated cardiomyocytes from CD38KO mice show increased calcium transient amplitude in response to caffeine compared to WT cells but the time decay constants are not different between both groups. Each point in the graphs represents the average value for all the cells recorded for each mice (N=4 mice/group, n=8 and 9 for WT and CD38KO groups respectively). (c) Representative immunoblottings of whole cell lysates from hearts of WT, CD38KO and CD38CI (catalytically inactive) mice and its corresponding quantification (N= 4, 5 and 5, for WT, CD38KO and CD38CI groups). SERCA2a expression in the heart was upregulated in both, CD38KO and CD38CI groups compared to WT mice. Data are analyzed by unpaired two-sided t-test, *P<0.05.
