FIGURE 1.
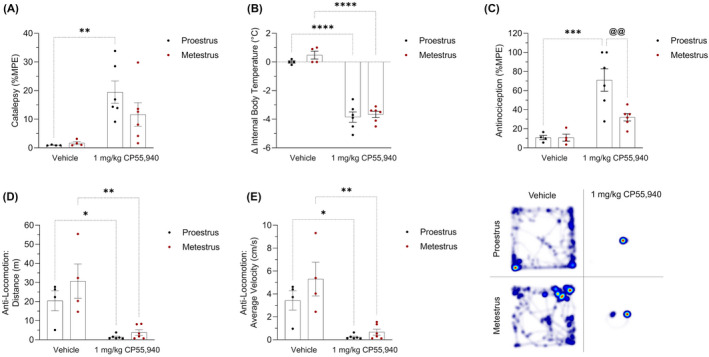
Acute tetrad effects in female mice following treatment with CP55,940. Female C57BL/6 mice aged 6–12 weeks in either proestrus or metestrus were administered either 1 mg/kg CP55,940 or a vehicle solution i.p. (A) 10 min following the injections, mice underwent the ring holding assay to measure their cataleptic response. All catalepsy data are expressed as %MPE (MPE = 60 s). (B) 15 min‐post injections, a rectal thermometer was used to measure internal body temperature. Temperatures are recorded as °C. (C) 20 min after injections, thermal antinociception was measured using the tail flick latency assay. Latencies are presented as %MPE (MPE = 20 s). 25 min following injections, mice were placed in the open‐field test evaluating treatment‐induced locomotion. (D) Distance travelled is reported in m. (E) Average velocity is recorded in cm/s. (F) Representative heat map images illustrating locomotion in the open‐field test. (A–E) Each dot on the graphs represent individual mice or n. Bars on the graphs indicate means ± SEM, where n = 4 (vehicle) and n = 6 (1 mg/kg CP55,940). All means were compared between 1 mg/kg CP55,940 and vehicle groups within estrus cycle phases, as well as between proestrus and metestrus groups within treatments. Significance was calculated using a two‐way ANOVA followed by Tukey's post hoc analyses. */**/***/****p < .05/.01/.001/.0001 compared with vehicle within estrus cycle phase. @@ p < .01 compared with proestrus within treatment
