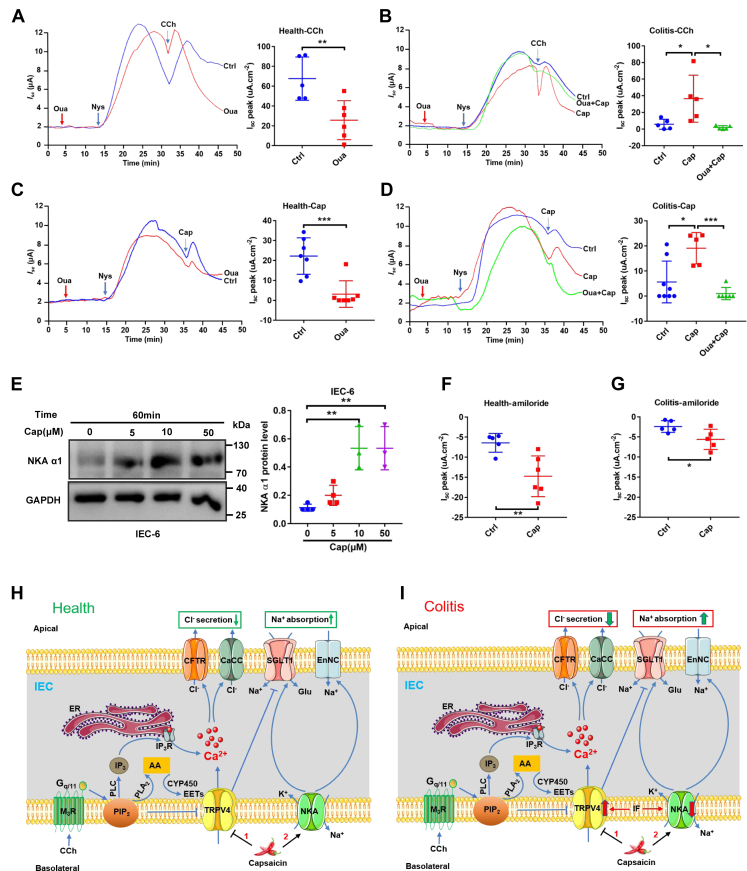
Figure 7.
Capsaicin stimulation of NKA and ENaC activities in the colonic epithelia of healthy and colitis mice and the proposed mechanisms of capsaicin action on intestinal epithelial ion transports.A, representative time courses and summary data showing the effect of CCh (100 μM, n = 5) on distal colonic Isc across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of ouabain (Oua, 100 μM, n = 5) in healthy mice. B, representative time courses and summary data showing the effect of CCh on distal colonic Isc (n = 5) across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of ouabain in colitic mice with or without capsaicin pretreated. C, representative time courses and summary data showing the effect of capsaicin (Cap, 30 μM, n = 7) on distal colonic Isc across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of Oua (n = 7) in healthy mice. D, representative time courses and summary data showing the effect of Cap on distal colonic Isc across the basolateral membrane in apically permeabilized epithelia in the absence or the presence of Oua (n = 6) in colitic mice with or without capsaicin pretreated. E, Western blot showing that Cap induced NKAα1 in IEC-6 cells (n = 3 independent experiments). F, summary data showing the distal colonic Isc in response to amiloride (100 μM, n = 5) in the absence or the presence of capsaicin (30 μM, n = 5) in healthy mice. G, summary data showing the colonic Isc (n = 5) in response to amiloride in colitic mice with or without capsaicin pretreated. CCh and ouabain were added to serosal side. Amiloride was added to mucosal side, but capsaicin was added to both sides. The data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 were performed by Student’s t test. H, in health, after the PIP2 suppression on basolateral TRPV4 channels is relieved by the activation of PLC and PLA2 in cholinergic signaling pathway, Ca2+ enters via TRPV4 channels and ER/Ca2+ releases via IP3R. Cytosolic Ca2+ stimulates Ca2+-dependent Cl- secretion through apical CFTR and CaCC. TRPV4 channels could also be activated by their endogenous ligands CYP450 metabolites produced from AA/CYP450 pathway. Capsaicin attenuates Cl- secretion but stimulates Na+ absorption by blocking TRPV4 channels but stimulating NKA activity on the basolateral side of intestinal epithelial cells (IEC). I, in colitis, the expression and function of TRPV4 channels are enhanced but NKA activities are reduced by inflammatory factors, the Cl- secretion are increased, and Na+ absorption are decreased. Capsaicin ameliorates colitis and reduces diarrhea by: (1) suppressing hyperactivation of basolateral TRPV4 channels to reduce intestinal Cl- secretion and (2) blocking basolateral TRPV4 channels and stimulating NKA activity to increase intestinal Na+ absorption. AA, arachidonic acid; CaCC, Ca2+-activated Cl- channels; CFTR, cystic fibrosis transmembrane conductance regulator; CYP450, cytochrome P450; [Ca2+]cyt, cytosolic Ca2+ concentrations; ENaC, epithelial Na+ channels; ER, endoplasmic reticulum; IP3R, IP3 receptor; IF, inflammatory factors; NKA, Na+/K+-ATPase; SGLT1, Na+-glucose cotransporter 1; TRPV, transient receptor potential vanilloid.