Abstract
Background
There are no data comparing a regular diet with a restricted diet after endoscopic polypectomy in patients with colorectal polyps. The current guidelines also did not provide the detailed information of dietary patterns after polypectomy. In this study, we aimed to evaluate the safety and efficacy of different diets on post-polypectomy outcomes.
Methods
A total of 302 patients with colorectal polyps who underwent polypectomy were prospectively enrolled between March 2019 and December 2019 in Nanfang Hospital (Guangzhou, China). Enrolled patients were then randomly assigned to a regular diet group or a restricted diet group after polypectomy. The study is a non-inferior design and the primary end point was the post-operative adverse events (AE) rate. Secondary end points included length of stay (LOS) and hospitalization cost.
Results
Among all the included patients, 148 patients received a restricted diet and 154 patients received a regular diet after polypectomy. A total of 376 polyps were removed, with 183 polyps in the restricted diet group and 193 polyps in the regular diet group. Shorter LOS (4.0 ± 1.4 vs 4.8 ± 1.7, P < 0.001) and lower hospitalization costs (7,701.63 ± 2,579.07 vs 8,656.05 ± 3,138.53, P = 0.001) were observed in the regular diet group. In particular, there was no significant difference in 3-day AE rates between the restricted diet and the regular diet group (1.35% [2/148] vs 2.60% [4/154], P = 0.685). Subgroup analysis looking at the number of polyps removed in each patient and different treatment modalities also showed similar findings.
Conclusion
Regular diet should be recommended after polypectomy for polyps <20 mm as it can shorten LOS and save hospitalization costs.
Keywords: colorectal polyps, polypectomy, regular diet, restricted diet, adverse events
Introduction
With the development of colorectal cancer screening programs, the detection rate of colorectal polyps has been increasing in China [1]. Recently, a cancer screening program in urban China showed the detection rates of advanced adenomas, non-advanced adenomas, and hyperplastic polyps were 3.07%, 8.17%, and 4.33%, respectively [2]. Polypectomy has been widely accepted as the standard method for removal of colorectal polyps. Hot snare polypectomy (HSP) and endoscopic mucosal resection (EMR) represent the most common modalities of polypectomy. The standard of care in Western countries for post-polypectomy patients is going home on the day of the procedure and they are told to eat a regular diet [3]. In China, patients are told to not consume food for 24 h after HSP or EMR to prevent possible post-operative adverse events (AE) such as post-polypectomy bleeding (PPB) and delayed perforation. However, there is no high evidence to support the idea of eating or fasting. Whether a patient should be kept at nothing by mouth or not still remains unclear. Previous research has shown that the potential risk factors for PPB are either polyp-related factors or patient-related factors. This includes polyp size, proximal location, endoscopist's experience, hypertension, and the use of anticoagulant [4–8]. Zhang et al. also analysed 5,600 patients who underwent polypectomy and found that polyps with a size of >10 mm and certain pathology of colonic polyps (especially Peutz-Jeghers) were significant risk factors for PPB [9]. As for delayed perforation, risk factors include attempted en bloc snare excision for lesions of >25 mm, high-grade dysplasia/early cancer, and transverse colon location [10]. However, these risk factors are inconsistent among studies and there is no evidence to support the impact of post-procedural diet on PPB or delayed perforation. Therefore, we carried out this randomized–controlled study to compare different diets on post-polypectomy outcomes.
Patients and methods
Patients
We prospectively recruited patients who were diagnosed with colorectal polyps and underwent polypectomy between March 2019 and December 2019 in the Department of Gastroenterology in Nanfang Hospital (Guangzhou, China). The inclusion criteria were patients aged 18–75 years who underwent polypectomy including high frequency electrotomy and EMR with a polyp size of <20 mm and fewer than five polyps removed who provided consent. Patients were excluded if they had severe co-morbidities such as cancer and organ failure, were pregnant, received other procedures, or had anal diseases. All the enrolled patients were randomly assigned to restricted diet group or regular diet after polypectomy. A random-number table was used to generate the random allocation sequence. The patients in the restricted diet group fasted for 24 h and then transitioned from liquid diet to regular diet within 48 h. The patients in the regular diet group were told to eat food as they would normally without fasting after polypectomy. Standard demographic and clinicopathologic data were collected including gender, age, body mass index (BMI), medical history, polyp site, polyp number, polyp size, polypectomy-related details, post-operative AE, length of stay (LOS), and hospitalization costs. Polypectomy-related details included polypectomy method, procedure time, intraprocedural bleeding, intraprocedural perforation, and prophylactic clip closure or not. Post-operative AE included PPB, post-polypectomy electrocoagulation syndrome (PPES), and delayed perforation.
Study design
The study was conducted at the Department of Gastroenterology. It was designed as a randomized–controlled trial performed in a parallel manner. The primary end point of this study was the post-polypectomy AE rate, and the secondary end points included LOS and hospitalization costs. All the participants received 3-day, 7-day, and 14-day follow-ups to evaluate post-operative AE. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and the protocol was approved by the Institutional Review Board of Southern Medical University. This clinical trial was also registered in the Chinese Clinical Trail Registry center (ChiCTR1900021673).
Interventions (restricted diet and regular diet)
Patients in the restricted diet group fasted and were given intravenous infusion of 5% glucose and sodium chloride for the first 24 h. If there were no complications or discomfort during the fasting, patients were given soup for the next 24 h. Finally, they were gradually transitioned from soup to a regular diet in the third 24 h.
Patients in the regular diet group directly received regular diet such as porridge, noodles, and rice after polypectomy. Meanwhile, all the patients in the two groups were given proton-pump inhibitors for 3 days intravenously.
Definition of PPB
According to the European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline, PPB is bleeding occurring after the procedure, ≤30 days post-polypectomy, that results in an unplanned medical presentation such as emergency department visit, hospitalization, or re-intervention (repeat endoscopy, angiography, or surgery).
Definition of PPES
PPES occurs as a result of transmural thermal injury to the bowel wall, with serosal inflammation and localized peritonitis [11]. Patients typically present within hours to days after polypectomy with fever, localized abdominal pain, and absence of perforation on radiographic imaging.
Definition of delayed perforation
Delayed perforation was defined as cases in which perforation had not been detected during and just after completion of polypectomy, but subsequent endoscopy showed perforation and radiography showed free air after polypectomy.
Sample-size calculation
Delayed bleeding was reported to be 1% of patients with sessile polyps of <20 mm in size following HSP [3, 12] and was found to be 6% of 1,039 patients with EMR [13]. An average delayed bleeding rate of 3.5% was used. In addition, PPES and delayed perforation ranges between 0.2% and 0.5% [11, 14]. Based on the above data, we assumed a total AE rate of 4% for both groups in our study and the non-inferiority margins for regular diet compared with restricted diet were defined at 4% in absolute risk difference, which would ensure that the regular diet results would not exceed the AE rate of 8%. For an α-error level of 0.05 (one-sided) and a β-error level of 0.20, 145 patients in each group were required, for a total of 290 patients (PASS 11.0 software, NCSS, Kaysville, USA). Assuming that ∼10% of patients may be excluded, the target sample size was set at 320 patients in total.
Statistical analysis
Continuous data are presented as mean and standard deviation (SD) or median and range. Categorical data are presented as number and percentage. For the statistical analysis of the primary end point, the incidence of AE between the two groups was compared using two-tailed Fisher’s exact test or Pearson’s chi-squared test. For the statistical analysis of the secondary end points, t-test of two independent samples was used. We also calculated the odds ratio (OR) and associated 95% confidence interval. All tests for significance were two-tailed and P-values of <0.05 were considered statistically significant in all cases. All analyses were performed using SPSS 19.0 (IBM, Armonk, NY, USA).
Results
Recruitment and participant flow
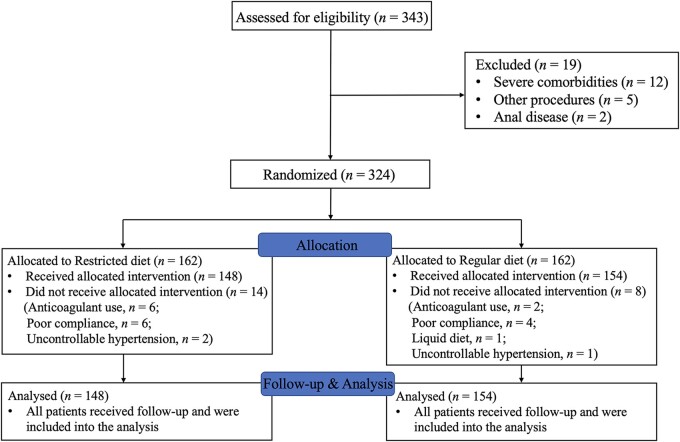
A total of 343 patients were recruited into the study and underwent polypectomy, with 376 polyps resected between March 2019 and December 2019. With regard to group allocation, 324 patients were then randomly assigned to a restricted diet or a regular diet, with 162 patients in each group. However, 22 patients were excluded or dropped out because of poor compliance, oral anticoagulant use, and uncontrollable hypertension. Therefore, 148 patients in the restricted diet group and 154 patients in the regular diet group were eventually enrolled into the analysis (Figure 1).
Figure 1.
Flow diagram of the study
Baseline data
The average age of the patients was 48.1 ± 10.4 years (range, 20–70 years), with a slight male predominance (207/302, 68.54%) (Table 1). The BMI was 23.25 ± 3.47 kg/m2. There were 376 polyps resected in the 302 patients with a single polyp removed in 223 patients (73.84%) and multiple polyps in 79 patients (26.16%). The left colon was the most common location of polyps, such as the sigmoid colon (35.11%) and the rectum (18.35%). The mean polyp size was 8.9 ± 3.6 mm (range, 3–20 mm). Among all the polypectomy procedures, HSP was performed for 214 lesions (70.86%) and EMR was applied in 69 lesions (22.85%). The mean operation time for all the patients was 16.1 ± 6.0 min. In addition, endoscopic clip closure of the defect was made in 241 lesions.
Table 1.
Characteristics of patients who underwent endoscopic polypectomy in restricted diet group and regular diet group
| Characteristic | Total (n = 302) | Restricted diet (n = 148) | Regular diet (n = 154) | P-value |
|---|---|---|---|---|
| Male, n (%) | 207 (68.54) | 106 (71.62) | 101 (65.58) | 0.259 |
| Age, years, mean ± SD | 48.1 ± 10.4 | 47.5 ± 10.6 | 48.8 ± 10.2 | 0.282 |
| BMI, kg/m2, mean ± SD | 23.25 ± 3.47 | 23.58 ± 3.73 | 22.92 ± 3.19 | 0.100 |
| Polyp number, n (%) | 0.477 | |||
| Single polyp | 223 (73.84) | 112 (75.68) | 111 (72.08) | |
| Multiple polyps (2) | 79 (26.16) | 36 (24.32) | 43 (27.92) | |
| Polyp location, n (%) | n = 376 | n = 183 | n = 193 | 0.252 |
| Caecum | 17 (4.52) | 12 (6.56) | 5 (2.59) | |
| Ascending colon | 40 (10.64) | 20 (10.93) | 20 (10.36) | |
| Transverse colon | 73 (19.41) | 38 (20.77) | 35 (18.13) | |
| Descending colon | 45 (11.97) | 17 (9.29) | 28 (14.51) | |
| Sigmoid colon | 132 (35.11) | 66 (36.07) | 66 (34.20) | |
| Rectum | 69 (18.35) | 30 (16.39) | 39 (20.21) | |
| Polyp size, mm, mean ± SD | 8.9 ± 3.6 | 9.8 ± 3.8 | 8.1 ± 3.2 | <0.001 |
| Polypectomy modality, n (%) | 0.316 | |||
| HSP | 214 (70.86) | 99 (66.89) | 115 (74.68) | |
| EMR | 69 (22.85) | 39 (26.35) | 30 (19.48) | |
| HSP & EMR | 19 (6.29) | 10 (6.76) | 9 (5.84) | |
| Operation time, min, mean ± SD | 16.1 ± 6.0 | 16.0 ± 5.8 | 16.1 ± 6.2 | 0.757 |
BMI, body mass index; EMR, endoscopic mucosal resection; HSP, hot snare polypectomy; SD, standard deviation.
Overall outcomes
The overall rate of post-polypectomy AE was 1.99% (n = 6) (Table 2). There were two cases of PPB in the restricted diet group. One case of PPB and three cases of PPES occurred in the regular diet group during the 3-day follow-up. There was no significant difference in the AE rate observed between the two groups (1.35% vs 2.60%, P = 0.685). All the PPB was successfully managed using endoscopic hemostasis. No delayed perforation was reported in the present study and no AE was observed at the time of 7-day and 14-day follow-ups. Our data demonstrated that LOS (4.0 ± 1.4 vs 4.8 ± 1.7 days, P < 0.001) and LOS after operation (2.3 ± 1.1 vs 2.8 ± 1.2 days, P < 0.001) were both much shorter in the regular diet group and hospitalization costs in the regular diet group were also lower (7,701.63 ± 2,579.07 vs 8,656.05 ± 3,138.53 RMB, P = 0.001).
Table 2.
Clinical outcomes of patients in restricted diet group and regular diet group after polypectomy
| Characteristic | Total (n = 302) | Restricted diet (n = 148) | Regular diet (n = 154) | P-value |
|---|---|---|---|---|
| 3-day adverse events, n (%) | 6 (1.99) | 2 (1.35) | 4 (2.60) | 0.685 |
| Length of stay, days, mean ± SD | 4.4 ± 1.6 | 4.8 ± 1.7 | 4.0 ± 1.4 | <0.001 |
| Length of stay after polypectomy, days, mean ± SD | 2.5 ± 1.2 | 2.8 ± 1.2 | 2.3 ± 1.1 | <0.001 |
| Hospitalization cost, ¥, mean ± SD | 8,169.36 ± 2,901.74 | 8,656.05 ± 3,138.53 | 7,701.63 ± 2,579.07 | 0.001 |
SD, standard deviation.
Subgroup analysis
Subgroup analysis of patients with a single polyp removed showed that the difference in the AE rate was not statistically significant between the regular diet group and the restricted diet group (3.60% vs 0.89%, P = 0.212), while there were significantly lower LOS (4.1 ± 1.4 vs 4.9 ± 1.7 days, P < 0.001) and hospitalization costs (7,760.92 ± 2,535.98 vs 8,646.50 ± 2,817.98 RMB, P = 0.004) in the regular diet group. Subgroup analysis of patients with multiple polyps showed no difference in hospitalization costs between the two groups whereas the LOS in the regular diet group was lower, and the data are presented in Table 3.
Table 3.
Subgroup analysis of patients with single polyp or multiple polyps in restricted diet group and regular diet group
| Characteristic | Single polyp (n = 223) |
Multiple polyps (n = 79) |
||||
|---|---|---|---|---|---|---|
| Restricted diet (n = 112) | Regular diet (n = 111) | P-value | Restricted diet (n = 36) | Regular diet (n = 43) | P-value | |
| Male, n (%) | 78 (69.64) | 70 (63.06) | 0.298 | 28 (77.78) | 31 (72.09) | 0.563 |
| Age, years, mean ± SD | 46.6 ± 9.9 | 47.4 ± 10.1 | 0.559 | 50.1 ± 12.2 | 52.3 ± 9.7 | 0.397 |
| Operation time, min, mean ± SD | 15.4 ± 5.5 | 15.49 ± 4.91 | 0.945 | 18.1 ± 6.5 | 17.6 ± 8.5 | 0.394 |
| Adverse events, n (%) | 1 (0.89) | 4 (3.60) | 0.212 | 1 (2.78) | 0 (0.00) | 0.456 |
| Hospitalization cost, ¥, mean ± SD | 8,646.50 ± 2,817.98 | 7,760.92 ± 2,535.98 | 0.004 | 8,685.78 ± 4,023.18 | 7,548.56 ± 2,711.82 | 0.173 |
| Length of stay, days, mean ± SD | 4.9 ± 1.7 | 4.1 ± 1.4 | < 0.001 | 4.6 ± 1.6 | 3.7 ± 1.3 | 0.020 |
| Polypectomy modality, n (%) | 0.073 | 0.682 | ||||
| HSP | 77 (68.75) | 88 (79.28) | 22 (61.11) | 27 (62.79) | ||
| EMR | 35 (31.25) | 23 (20.72) | 4 (11.11) | 7 (16.28) | ||
| HSP & EMR | 0 (0.00) | 0 (0.00) | 10 (27.78) | 9 (20.93) | ||
| Polyp size, mm, mean ± SD | 9.7 ± 3.7 | 8.1 ± 3.1 | < 0.001 | 10.1 ± 4.0 | 8.1 ± 3.5 | 0.030 |
| Endoscopic clip closure, n (%) | 98 (87.50) | 84 (75.68) | 0.023 | 31 (86.11) | 28 (65.12) | 0.033 |
EMR, endoscopic mucosal resection; HSP, hot snare polypectomy; SD, standard deviation.
We also looked at a subgroup analysis of patients who underwent different modalities of polypectomy (HSP and EMR) (Table 4). In the subgroup of HSP, patients taking a regular diet had shorter LOS (4.1 ± 1.4 vs 5.0 ± 1.7 days, P < 0.001) and lower hospitalization costs (7,529.34 ± 2,754.81 vs 8,727.83 ± 3,322.20 RMB, P = 0.001); meanwhile, there was no significant difference in the AE rate between the regular diet group and the restricted diet group (2.61% vs 1.01%, P = 0.626). In the subgroup of EMR, we also observed no significant difference in the AE rate. However, our results showed no difference in LOS and hospitalization costs between the two groups in the subgroup of EMR. Furthermore, multivariate analysis concerning the risk factors of AE in post-polypectomy patients was also analysed, which revealed that diet was not a risk factor (Table 5).
Table 4.
Subgroup analysis of patients who underwent HSP or EMR in restricted diet group and regular diet group
| Characteristic | HSP (n = 214) |
EMR (n = 69) |
||||
|---|---|---|---|---|---|---|
| Restricted diet (n = 99) | Regular diet (n = 115) | P-value | Restricted diet (n = 39) | Regular diet (n = 30) | P-value | |
| Male, n (%) | 72 (72.73) | 75 (65.22) | 0.238 | 28 (71.79) | 20 (66.67) | 0.646 |
| Age, years, mean ± SD | 47.7 ± 10.4 | 48.3 ± 10.3 | 0.678 | 45.8 ± 10.3 | 49.1 ± 10.5 | 0.193 |
| Operation time, min, mean ± SD | 16.2 ± 6.0 | 15.2 ± 4.8 | 0.166 | 14.8 ± 4.2 | 18.3 ± 9.1 | 0.113 |
| Adverse events, n (%) | 1 (1.01) | 3 (2.61) | 0.626 | 1 (2.56) | 1 (3.33) | 1.000 |
| Length of stay, days, mean ± SD | 5.0 ± 1.7 | 4.1 ± 1.4 | < 0.001 | 4.4 ± 1.7 | 3.8 ± 1.4 | 0.119 |
| Hospitalization cost, ¥, mean ± SD | 8,727.83 ± 3,322.20 | 7,529.34 ± 2,754.81 | 0.001 | 8,627.20 ± 2,721.36 | 8,217.07 ± 2,039.90 | 0.804 |
| Size, mm, mean ± SD | 9.6 ± 4.0 | 7.5 ± 3.2 | < 0.001 | 10.0 ± 3.4 | 9.5 ± 3.0 | 0.511 |
EMR, endoscopic mucosal resection; HSP, hot snare polypectomy; SD, standard deviation.
Table 5.
Multivariate analysis representing risk factors of adverse events in 302 patients after polypectomy
| Variable | B | SE | Wald | P-value | OR | 95% CI of OR value |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 0.052 | 0.045 | 1.349 | 0.245 | 1.053 | 0.965 | 1.150 |
| Polyp number | –0.970 | 1.140 | 0.724 | 0.395 | 0.379 | 0.041 | 3.543 |
| Diet group | –0.624 | 0.879 | 0.504 | 0.478 | 0.536 | 0.096 | 3.000 |
| Operating time | 0.047 | 0.060 | 0.619 | 0.431 | 1.049 | 0.932 | 1.180 |
B, beta coefficient; SE, standard error; OR, odds ratio; CI, confidence interval.
Discussion
Risk factors for delayed colonic PPB and perforation have been evaluated by many studies. However, we did not find any data about the impact of diet on post-polypectomy complications. Current guidelines also did not point out what kind of diet should be taken for patients after polypectomy. In the present study, we compared a regular diet with a restricted diet to evaluate the clinical effect on post-polypectomy AE. As demonstrated by our data, patients on a regular diet were non-inferior to a restricted diet in terms of AE and had a decreased LOS and hospitalization cost.
Colonoscopic polypectomy is widely used to remove colorectal polyps and has proven to reduce the incidence and mortality of colorectal cancer [15]. However, complications of polypectomy are encountered in clinical practice. Endoscopic resection of colorectal lesions is associated with a low incidence of significant complications, most commonly PPB, PPES, and delayed perforation. The key risk factors of PPB identified in previous studies include a lesion size of >30 mm, proximal colon location, and presence of major co-morbidities [7–10, 16]. As for delayed perforation, risk factors include attempted en bloc snare excision for lesions of >25 mm, high-grade dysplasia/early cancer, and transverse colon location [10]. Risk factors for PPES include right colonic resection, polyp size of >10 mm, hypertension, and non-polypoidal lesion morphologies [11]. Studies have not looked at the effect of different diets on AE after polypectomy. In the present study, we provided the comparative data about the two different diet models. The AE rates of two groups were similar and regular diet did not increase the AE rate, showing that there was no necessity for fasting. To our knowledge, most hospitals in China still adopt a restricted diet after polypectomy to prevent possible complications. However, in most countries in the world, colonoscopy, including polypectomy, is a same-day procedure and no patients will be admitted after the procedure. As shown by our data, the patients did not benefit from fasting; on the contrary, regular diet was associated with a reduction in LOS and hospitalization costs. Similar results were seen on the subgroup analysis looking at different modalities. It should be noted that our study only enrolled patients with polyps of <20 mm in maximum diameter, which may reduce the AE rate of polypectomy. In order to reduce the potential risk and protect the participants, patients with polyps of <20 mm were enrolled. However, this well-designed study provided a different point of view, which challenged our traditional opinion and practice in China. Further studies are needed to look at the effects of different diets on larger-sized polyps and endoscopic submucosal dissection (ESD).
There are a few limitations to this paper. First, this is a single-center study, which may limit the generalizability of the study results. Second, the study did not recruit patients with polyps of >20 mm in size, which reduced the AE rate of the study and made it difficult to detect the significant difference. However, our study is the first well-designed study comparing the clinical effectiveness of different diets on post-polypectomy AE, which provides a new insight about our clinical management after polypectomy. It is time to change Chinese traditional practice and adopt a regular diet for post-polypectomy patients based on this study. Moreover, our data also confirmed that the rate of AE is low and that hospitalizing such patients is not necessary.
In conclusion, a regular diet rather than restricted diet should be taken after polypectomy, which can shorten LOS and save hospitalization costs without increasing the risk of post-operative complications.
Authors’ Contributions
S.L. and Y.L. designed the study. Y.L. made the analysis and wrote the draft. R.H., S.H., L.X., B.C., and J.F. recorded the data. Y.W., Y.B., Z.H., and Z.W. provided the cases. R.M. and S.L. helped to revise the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the Science and Technology Planning Project of Guangdong Province [grant number 2017A020215139] and Guangdong Gastrointestinal Disease Research Center [grant number 2017B020209003].
Conflict of Interest
None declared.
References
- 1. Chi Z, Lin Y, Huang J. et al. Risk factors for recurrence of colorectal conventional adenoma and serrated polyp. Gastroenterol Rep 2022;10:goab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen H, Li N, Ren J. et al. ; Group of Cancer Screening Program in Urban China (CanSPUC). Participation and yield of a population-based colorectal cancer screening programme in China. Gut 2019;68:1450–7. [DOI] [PubMed] [Google Scholar]
- 3. Reumkens A, Rondagh EJA, Bakker CM. et al. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol 2016;111:1092–101. [DOI] [PubMed] [Google Scholar]
- 4. Choung BS, Kim SH, Ahn DS. et al. Incidence and risk factors of delayed postpolypectomy bleeding: a retrospective cohort study. J Clin Gastroenterol 2014;48:784–9. [DOI] [PubMed] [Google Scholar]
- 5. Gandhi S, Narula N, Mosleh W. et al. Meta-analysis: colonoscopic post-polypectomy bleeding in patients on continued clopidogrel therapy. Aliment Pharmacol Ther 2013;37:947–52. [DOI] [PubMed] [Google Scholar]
- 6. Heldwein W, Dollhopf M, Rösch T. et al. ; Munich Gastroenterology Group. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy 2005;37:1116–22. [DOI] [PubMed] [Google Scholar]
- 7. Rutter MD, Nickerson C, Rees CJ. et al. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy 2014;46:90–7. [DOI] [PubMed] [Google Scholar]
- 8. Watabe H, Yamaji Y, Okamoto M. et al. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp-related factors and patient-related factors. Gastrointest Endosc 2006;64:73–8. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Q, An S. L, Chen Z. y. et al. Assessment of risk factors for delayed colonic post-polypectomy hemorrhage: a study of 15553 polypectomies from 2005 to 2013. PLoS One 2014;9:e108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferlitsch M, Moss A, Hassan C. et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270–97. [DOI] [PubMed] [Google Scholar]
- 11. Cha JM, Lim KS, Lee SH. et al. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy 2013;45:202–7. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto M, Kato M, Oba K. et al. Multicenter randomized controlled study to assess the effect of prophylactic clipping on post-polypectomy delayed bleeding. Dig Endosc 2016;28:570–6. [DOI] [PubMed] [Google Scholar]
- 13. Burgess NG, Williams SJ, Hourigan LF. et al. A management algorithm based on delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014;12:1525–33. [DOI] [PubMed] [Google Scholar]
- 14. Ma MX, Bourke MJ.. Complications of endoscopic polypectomy, endoscopic mucosal resection and endoscopic submucosal dissection in the colon. Best Pract Res Clin Gastroenterol 2016;30:749–67. [DOI] [PubMed] [Google Scholar]
- 15. Zauber AG, Winawer SJ, O'Brien MJ. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sawhney M, Salfiti N, Nelson D. et al. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy 2008;40:115–9. [DOI] [PubMed] [Google Scholar]