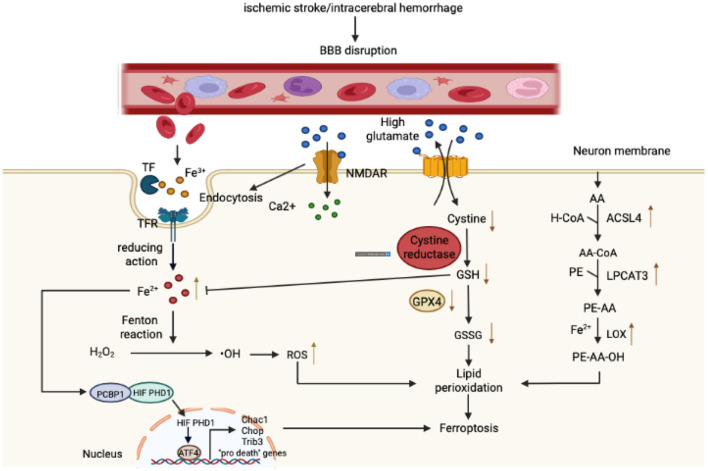
Figure 1.
Mechanism governing ferroptosis in stroke. With respect to iron metabolism, after ischemic or hemorrhagic stroke, the permeability of the blood-brain barrier (BBB) increases, causing a variety of components rich in Fe3+ in the bloodstream to infiltrate into the brain parenchyma. Fe3+ binds closely to transferrin (T) to form iron-containing TF, which then binds to TFR1 on the surface of brain cell membranes, enters cells through pinocytosis, and forms an endosome. In the acidic environment of an inclusion body, Fe3+ is released from TF, catalyzed by ferrous reductase to Fe2+, and transported to the cytoplasm through the corresponding transporter. Fe2+ then initiates the Fenton reaction to form reactive oxygen species (ROS) and also affects the catalytic activity of lipoxygenase (LOX). In addition, the Fe2+ in the cytoplasm can be absorbed by PCBP1 and iron is loaded on the iron-free form of HIF-PDH1. Iron-containing HIF-PDH1 together with other stimuli drives the activity of pro-ferroptosis ATF4 gene expression. With regard to amino acid metabolism, a high concentration of extracellular glutamate leads to a dysfunction in system XC−, a deficiency in intracellular cystine, depletion of GSH, and diminution in GPX4 activity. In addition, glutamate binds to its receptor (NMDAR), and the activation of NMDAR then further exacerbates iron uptake. Regarding lipid metabolism, as the main component of phospholipid membranes, arachidonic acid (AA) is esterified with phosphatidylethanolamine (PE) under the action of ACSL4 and LPCAT3 to synthesize PE-AA and is then catalyzed by LOX to PE-AA-OH. As shown in the figure, these three components together lead to lipid peroxidation and ferroptosis.