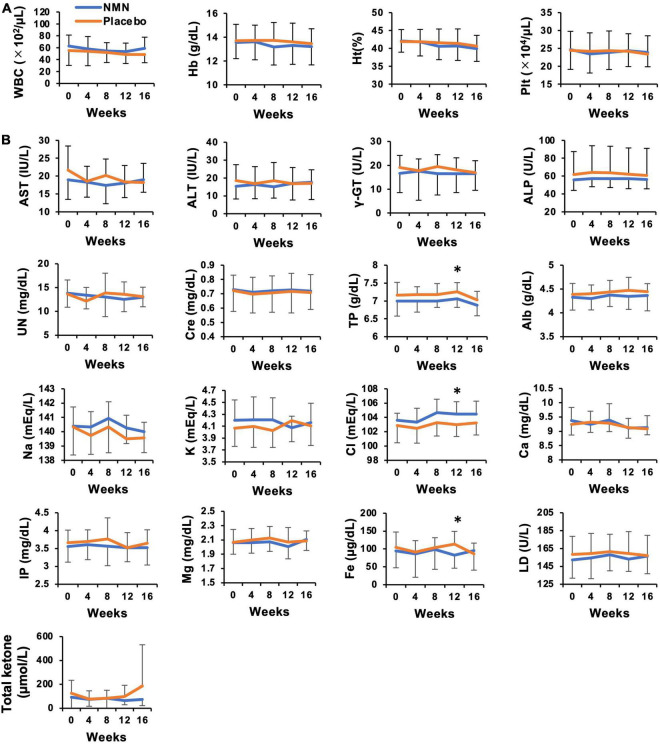
FIGURE 3.
No adverse event was observed in laboratory data for safety evaluation of NMN oral administration. Hematological (A) and biochemical (B) blood tests were performed every 4 weeks. Orange represents placebo group (n = 15 at 0, 4, 8 weeks, n = 14 at 12, 16 weeks) and blue represents NMN group (n = 15). Data are represented as mean ± SD. WBC, white blood cells; Hb, hemoglobin; Ht, hematocrit; Plt, platelets; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase; ALP, alkaline phosphatase; UN, urine nitrogen; Cre, serum creatinine; TP, total protein; Alb, albumin; Na, serum sodium; K, serum potassium; Cl, serum chloride; Ca, serum calcium; Mg, serum magnesium; Fe, serum iron; IP, inorganic phosphorus; LD, lactate dehydrogenase. Asterisk means statistical significance: p-value < 0.05.