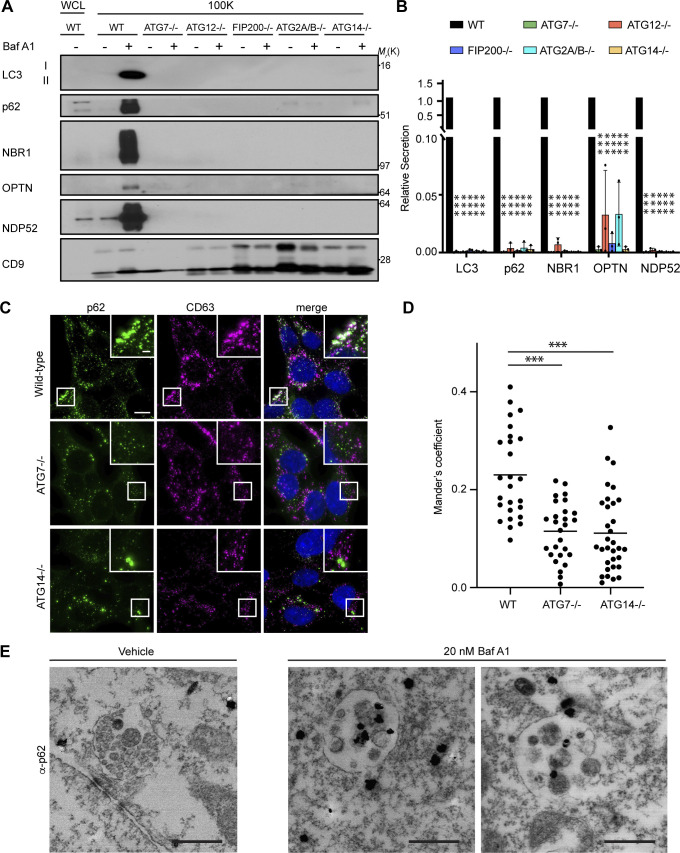
Figure 5.
Autophagy cargo receptor secretion during lysosome inhibition requires autophagosome formation. (A) Cell lysate (WCL; left) and 100,000 g EV fractions (100K; right) from serum-starved WT and ATG-deficient HEK293T cells treated with vehicle or 20 nM BafA1 for 16 h were blotted to detect LC3, autophagy cargo receptors, and CD9 (n = 4). (B) Quantification of the indicated proteins in EVP fractions from BafA1 treated cells relative to treated WT controls. Statistics were calculated by nonparametric one-way ANOVA coupled with Dunnett’s post hoc test (mean ± SEM; n = 4; ***, P < 0.005). (C) Representative fluorescence micrographs from serum-starved WT, ATG7−/−, and ATG14−/− cells treated with 20 nM BafA1 and immunostained for endogenous p62 (green), CD63 (magenta), and LC3. Immunofluorescence micrographs of endogenous LC3 and CD63 from these exact cell samples and corresponding co-occurrence data can be found in Fig. S4, C–E. Scale bar, 10 μm; inset scale bar, 2 μm. (D) Scatter plot of Mander’s coefficients for the co-occurrence of p62 and CD63 in C. Statistics were calculated by nonparametric one-way ANOVA with Dunnett’s post hoc test (mean ± SEM; WT n = 26; ATG7−/− n = 26, ATG14−/−, n = 32; ***, P < 0.005). (E) Representative images from TEM of late endosomes from vehicle or 20 nM BafA1 treated cells that were immunostained with anti-p62 primary antibody and detected using ultrasmall gold-conjugated secondary antibody with silver enhancement (scale bar, 500 nm). Source data are available for this figure: SourceData F5.