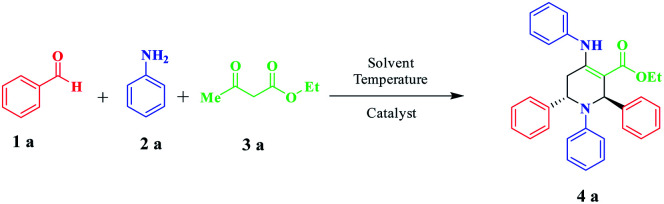
Optimization of the solvent for the synthesis of piperidine in the model reactiona.
| |||||
|---|---|---|---|---|---|
| Entry | Solvent | Catalyst (mol%) | Temp. (°C) | Time (h) | Yieldb (%) |
| 1 | H2O | 1.5 | 40 | 12 | 40 |
| 2 | EtOH | 1.5 | 40 | 2 | 94 |
| 3 | MeOH | 1.5 | 40 | 5 | 88 |
| 4 | EtOAc | 1.5 | 40 | 5 | 50 |
| 5 | CH3CN | 1.5 | 40 | 8 | 85 |
| 6 | Solvent free | 1.5 | 40 | 12 | 43 |
| 7 | DMF | 1.5 | 40 | 6 | 55 |
| 8 | THF | 1.5 | 40 | 12 | 70 |
| 9 | EtOH | — | 40 | 24 | Trace |
| 10 | EtOH | 0.5 | 40 | 8 | 58 |
| 11 | EtOH | 1 | 40 | 2 | 77 |
| 12 | EtOH | 2 | 40 | 2 | 95 |
| 13 | EtOH | 1.5 | RT | 12 | 70 |
| 14 | EtOH | 1.5 | 50 | 2 | 94 |
| 15 | EtOH | 1.5 | 60 | 1 : 45 | 90 |
| 16 | EtOH | 1.5 | 70 | 1 : 30 | 85 |
| 17 | EtOH | 1.5c | 40 | 24 | 0 |
| 18 | EtOH | 1.5d | 40 | 24 | Trace |
| 19 | EtOH | 1.5e | 40 | 12 | 75 |
Experimental conditions: benzaldehyde (2 mmol), aniline (2 mmol), methyl acetoacetate (1 mmol), and catalyst (1.5 mol%) in 5 mL of solvent at 40 °C.
Isolated yields.
Reaction was performed in the presence of MNPs as the catalyst.
Reaction was performed in the presence of MNPs@CNF as the catalyst.
Reaction was performed in the presence of MNPs@CNF@ATS–Co(ii) as the catalyst.
