Synthesis of various poly-substituted piperidine derivativesa.
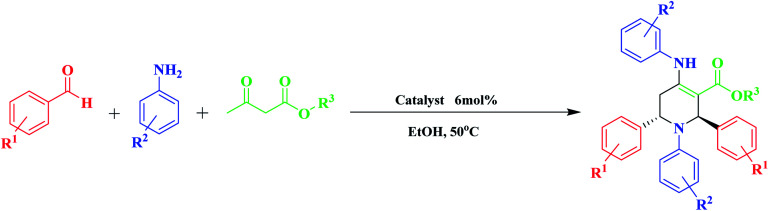
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | Product | Time (h) | Yieldb (%) | m.p./°C | Lit. m.p./°C [ref.] |
| 1 | H | H | Et | 4a | 2 | 92 | 173–174 | 174–175 [ref. 35] |
| 2 | H | H | Me | 4b | 2 | 94 | 169–170 | 169–171 [ref. 35] |
| 3 | H | 4-Br | Et | 4c | 3 | 87 | 198–199 | 196–198 [ref. 36b] |
| 4 | H | 4-Cl | Et | 4d | 3 | 83 | 201–202 | 202 [ref. 36c] |
| 5 | 4-OMe | 4-Cl | Me | 4e | 2.5 | 85 | 195–196 | 193–195 [ref. 36e] |
| 6 | 4-OMe | 4-Br | Me | 4f | 2.5 | 78 | 177–178 | 177–179 [ref. 20] |
| 7 | 2-NO2 | H | Me | 4g | 2 | 70 | 217–218 | 217–219 [ref. 35] |
| 8 | 4-NO2 | H | Me | 4h | 3 | 82 | 240 | 239–241 [ref. 36d] |
| 9 | 4-NO2 | H | Et | 4i | 3 | 87 | 249–250 | 247–250 [ref. 36d] |
| 10 | 4-Br | H | Me | 4j | 4 | 84 | 245–246 | 245–247 [ref. 36c] |
| 11 | 4-Br | 4-Cl | Me | 4k | 4 | 82 | 160–161 | 160–163 [ref. 36c] |
| 12 | 4-Cl | H | Me | 4l | 4 | 82 | 227 | 225–227 [ref. 36e] |
Reaction conditions: benzaldehyde (2 mmol), aniline (2 mmol), β-ketoester (1 mmol), and catalyst (1.5 mol%) in 5 mL of EtOH at 50 °C.
Isolated yield.
