Catalytic activity of the Zr@IL-Fe3O4 MNPs in the synthesis of dihydropyrano[3,2-c]chromenes under solvent-free conditionsa.
| Entry | Aldehyde | Time (min) | Yieldb (%) | M.P. (obsd) (°C) | M.P. (lit.) (°C) | Product |
|---|---|---|---|---|---|---|
| 1 |

|
10 | 96 | 254–256 | 255–256 (ref. 45) |

|
| 2 |

|
15 | 96 | 257–259 | 258–260 (ref. 46) |

|
| 3 |

|
15 | 92 | 247–249 | 246–248 (ref. 47) |
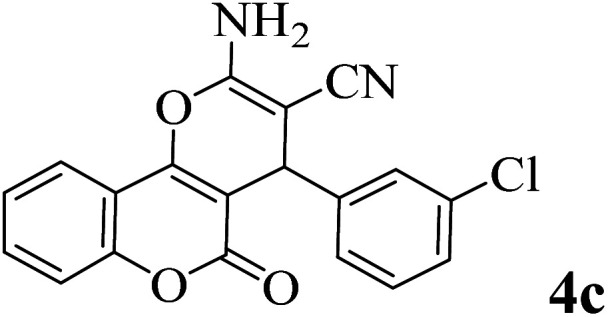
|
| 4 |

|
15 | 95 | 267–269 | 266–268 (ref. 48) |

|
| 5 |

|
15 | 92 | 248–251 | 252–254 (ref. 49) |

|
| 6 |

|
20 | 94 | 290–292 | 295–297 (ref. 48) |

|
| 7 |

|
15 | 92 | 255–257 | 258–260 (ref. 38) |

|
| 8 |

|
15 | 94 | 258–260 | 261–262 (ref. 45) |
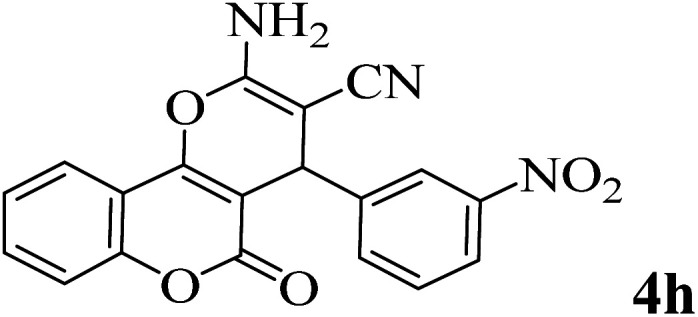
|
| 9 |

|
15 | 96 | 254–256 | 257–259 (ref. 38) |

|
| 10 |

|
15 | 92 | 285–287 | 289–290 (ref. 48) |

|
| 11 |

|
15 | 95 | 259–261 | 260–262 (ref. 47) |

|
| 12 |

|
20 | 92 | 263–265 | 266–267 (ref. 49) |

|
| 13 |
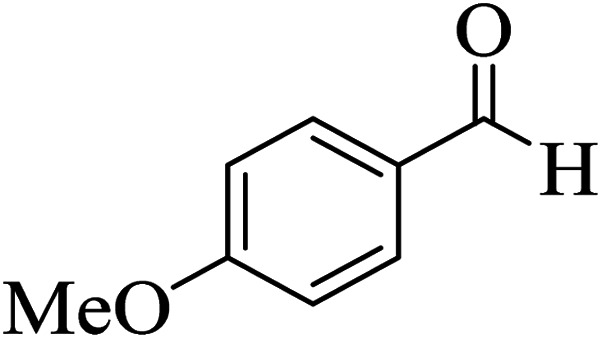
|
25 | 90 | 225–227 | 222–224 (ref. 45) |

|
| 14 |

|
25 | 91 | 252–254 | 251–253 (ref. 47) |

|
| 15 |

|
25 | 91 | 253–255 | 250–252 (ref. 50) |
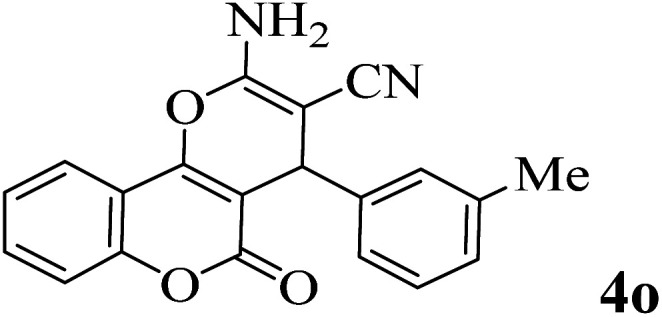
|
| 16 |

|
25 | 91 | 224–226 | 227–229 (ref. 51) |

|
| 17 |

|
25 | 91 | 252–254 | 252–253 (ref. 52) |

|
Reaction conditions: 4-hydroxycoumarin (1 mmol), malononitrile (1.2 mmol), aldehyde (1 mmol), Zr@IL-Fe3O4 MNPs (20 mg), solvent-free.
The yields refer to the isolated product.
