Abstract
Background
Data on the association between free-living daytime sunlight exposure and incident dementia are scarce. The objective is to evaluate whether the time spent in outdoor light is related to the dementia risk and to investigate whether the optimal duration varies with clinical parameters.
Methods
Data were from a prospective cohort of 362,094 UK Biobank participants. A questionnaire survey was conducted to investigate how many hours the participants spent outdoors on typical summer and winter days. A restricted cubic spline (RCS) was performed to explore the potential nonlinear relationship between sunlight exposure and the risk of dementia. We used multivariate Cox proportional hazard regression models to estimate the hazard ratios (HRs) for the associations between sunlight exposure and dementia outcomes, with the change points as a reference.
Results
After a median follow-up of 9.0 years, 4149 (1.15%) individuals were diagnosed with dementia. RCS showed a J-shaped relationship between time spent in outdoor light and the dementia risk, with the lowest risk at three change points (1.5 h/day on average, 2 h/day in summer, and 1 h/day in winter). Cox hazard regression models showed a marked increase in risk at low exposure (HR=1.287, 95%CI 1.094–1.515) but a relatively slow increase at higher exposure (HR=1.070, 95%CI 1.031–1.10). Results are more pronounced among participants over 60 years old, females, and those with exactly 7 h of sleep every night.
Conclusions
Sunlight exposure had a J-shaped association with dementia risk. Giving detailed guidance on sunlight exposure can effectively prevent dementia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02331-2.
Keywords: Dementia, Risk factors, Sunlight, Dose-response, UK Biobank
Background
Sunlight has important effects on vitamin D status, sleep rhythm, and other fields of human physiology; many of which are closely related to dementia [1–3]. As the main source of vitamin D in the human body, exposure to ultraviolet radiation (UV) in sunlight is essential for vitamin D synthesis [2]. Multiple longitudinal cohort studies indicated an association between low circulating vitamin D levels and an increased risk of dementia [2, 4, 5]. Besides, 44% of demented patients have sleep-wake disorders, such as difficulty falling asleep (insomnia), frequent awakening (sleep fragmentation), and nighttime confusion, which are often accompanied by diurnal changes in behavior and cognition [6]. Circadian dysrhythmias have further effects on neuroendocrine systems, including cognitive function and emotion. As the most essential “zeitgeber,” sunlight directly affects wakefulness, mood, and cognition, which is referred to as a non-image-forming (NIF) function [7]. Previous studies have also shown that depression is negatively correlated with sunlight exposure [3]. Patients with depression in a room with eastern light had a mean 3.67-day shorter hospital stay than those in west-facing rooms [8]. It has been proven that less sunlight increases the risk of cognitive decline in patients with depression by 2.58 times [9]. Moreover, bright light treatment (BLT) is considered as a promising non-drug intervention for dementia, since it has positive effects on behavioral and psychological symptoms of dementia, sleep, and circadian rhythms [1].
All those studies provided direct or indirect evidence for the hypothesis that sunlight may affect the risk of dementia. However, few epidemiological studies in the adult population-related these outcomes to daytime sunlight exposure. Previously, little information was available on the effects of natural sunlight on dementia. And as far as we know, the dose-response relationship has not been studied yet [10, 11]. It is worth noting that for individuals aged 4 to 64, there is no UK dietary reference value for vitamin D as they are assumed to get enough vitamin D from daylight exposure alone, which makes our research more meaningful [12–14]. Here, we conducted a large-scale cohort study on the longitudinal correlation between daytime light exposure and dementia-related outcomes in the British population. The aim of our study is to provide guidance on sunlight exposure to effectively prevent dementia in the targeted population living in high latitudes far from the equator.
Methods
Study design and participants
UK Biobank is a population-based prospective cohort study that recruited about 500,000 males and females aged 37 to 73 years from 2006 to 2010 [15]. The Ethics Committee permitted the UK Biobank from the Northwest Multi-Center Research Ethics Committee (Research Ethics Committee Reference: 16 /NW/0274). All participants assessed one of 22 assessment centers in England, Scotland, or Wales, covering various settings to provide socio-economic and ethnic heterogeneity and urban-rural mix. This ensures a wide distribution in all exposure ranges to detect the generalized correlation between baseline characteristics and health outcomes. After informed consent, participants accomplished a touch screen questionnaire, oral interview, and physical examination and reserved some biological samples.
Measurement of sunlight exposure
Participants reported how many hours they respectively spent the daylight outdoors on summer and winter typical days. Data was collected at baseline assessment (2006–2010). This section is defined as fields 1050 and 1060 in the UK Biobank database. Participants described a number using a touch-screen pad or selected integers that included “less than an hour a day,” “do not know,” or “prefer not to answer” in several options set in advance. If participants spent a lot of time outdoors, the average time they spent daily should be provided. Initial data preparation included deleting participants who reported “do not know” or “prefer not to answer” (n=33856) and redefining “less than an hour a day” as 0 (n=19865). Considering the effective daytime duration in the UK, extreme values larger than the typical day length in summer (16 h, n=253) and winter (8 h, n=5472) were excluded. To unify the outdoor light exposure time standard, the duration reported in winter and summer was averaged within the participants. We exclude people whose average time is more than twice the standard deviation for analysis. There is a strong correlation between the length of outdoor lighting reported in summer and winter (Pearson’s r=0.541, P<0.001), indicating that participants with more outdoor light time in summer also tend to spend more time in winter.
Covariates
The selection of covariates was based on the following criteria: demographic variables, exposure-related variables, and confounding variables associated with AD [16, 17]. The following variables were selected: age, sex, education, skin color, use of sun/UV protection, employment status, sleep duration, and pollution of air, fracture history, vitamin D supplement, hearing loss, smoking status, alcohol use, cardiovascular disease (CVD), total physical activity (TPA), and body mass index (BMI). The response of the skin to UV depends on the difference in skin color caused by the distribution of melanocytes caused by the size, volume, and keratinocytes rather than the difference in the number of melanocytes between races [18, 19]. Thus, we choose skin color rather than race as a covariate. Information on education (with or without a college or university degree), skin color (white or colored), use of sun/UV protection (never/rarely, yes, and do not go out in the sunshine), employment status (yes or no), fracture history in past 5 years (yes or no), vitamin D supplement (yes or no), hearing loss (yes or no), cigarette, and alcohol consumption (never, former, and current) was collected from the touchscreen questionnaire. Sleep duration was divided into short (<7 h per night), normal (7 h per night), and long (>7 h per night) [20]. Considering that high air pollution concentrations induce smog, which attenuates sunlight exposure, we used PM2.5 as one of the correction factors. CVD was collected from both the touchscreen questionnaire and verbal interview, including heart attack, angina, stroke, and hypertension. TPA was measured basing the revised International Physical Activity Questionnaire (IPAQ), including the frequency and duration of walking (Field 864 and 874), moderate (Field 884 and 894), and vigorous activity (Field 904 and 914) on a typical day/week over the past 4 weeks [21]. BMI was calculated from weight (kg) and standing height (meters) measured during the medical examination.
Measurement of outcome
Dementia syndromes were identified from the International Classification of Diseases, 9th and 10th revision (ICD9 and ICD10) codes of hospital inpatient admission data, provided by the UK Hospital Episode Statistics. Corresponding start dates and annual review dates were from Scottish Morbidity Records and Patient Episode Database. Participants followed up on the earliest diagnosis of dementia, the date of death, the date of the last data collection by general practitioners, or the time of the last hospitalization, whichever occurred first. According to the ICD, all-cause dementia was defined as code in ICD-9 codes 290, 290.4, 291.2, 294.1, 331.0-331.2, 331.5, and ICD-10 codes A81.0, F00, F01, F02, F03, F05.1, F10.6, G31.0, G31.1, G31.8, G30, and I67.3 (Additional file 1: Table S1). Additionally, dementia diagnoses were also retrieved from primary care statistical information utilizing reading codes (version 2 [Read v2] and version 3 [CTV3 or Read v3]) [22]. We excluded those who reported “dementia, Alzheimer’s disease, or cognitive impairment” in baseline to reduce the possibility of including prevalent cases in our analyses (n=34488). Missing participants without dementia outcomes were excluded (n=34572). The missing rate of the cohort is 6.88%.
Statistical analyses
If the distribution of variables is normal, t test was used to compare the average level of the incident dementia group and the no incident dementia group. Otherwise, the Mann-Whitney U test was used. Categorical variables were presented as numbers (percentages) and compared by the chi-square test. The dose-response relationship was flexibly modeled by the restricted cubic spline (RCS) with five knots to explore the potential nonlinear correlation between sunlight exposure and the risk of all-cause dementia [23]. With the change points as a reference, univariate and multivariate Cox proportional hazard regression models were fitted to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the sunlight exposure on dementia outcomes.
Series sensitivity analyses were conducted to test the robustness of our findings. First, we ran our survival models in white participants to check the potential role of skin color since most participants in this study are white. Second, we excluded the population with dementia events within the first 3 years and a further 10 years of follow-up to avoid potential reverse causation. To assess whether the association between time spent in outdoor light and risk of dementia differed across subpopulations, we examined potential effect modification by age at baseline (<60 and ≥60years old), age of dementia onset (early onset: <65 and late onset: ≥65 years old), and sex (male and female). Considering that limited light can lead to sleep disorders, we performed subgroup analysis according to sleep duration (<7 h, 7 h, and >7 h per night). We used the interaction between sunlight exposure time and each potential modifier to test the homogeneity across stratum-specific HRs.
Statistical analyses were completed using R 3.6.1 (R Foundation, Vienna, Austria). P values less than 0.05 were statistically significant.
Results
Characteristics of study participants
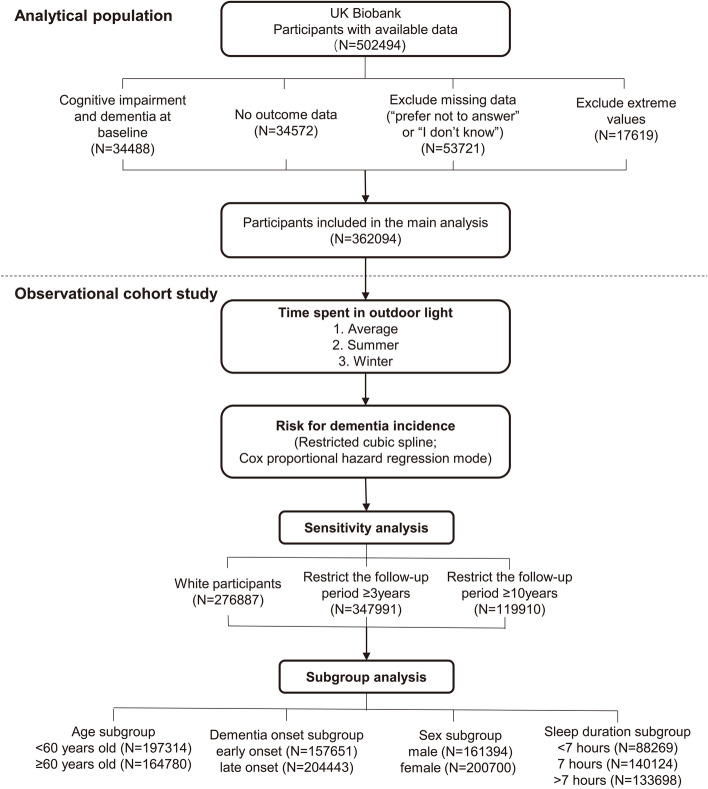
Figure 1 shows the flow chart. A total of 362,094 participants contributing 3,121,329 person-years at risk were included in the primary analyses. Among these participants, 4149 (1.15%) individuals were first-ever diagnosed with dementia during a median follow-up of 9.01 years (IQR 7.0–10.6). The baseline characteristics of the participants were summarized in Table 1 based on whether dementia occurred. Those who converted to dementia were more likely to be older, less educated, never or rarely used sun/UV protection, unemployed, without expected sleep duration, higher PM2.5 exposure, smokers, alcohol drinkers, and have more CVD diagnoses (all P<0.05). Notably, the average time spent in outdoor light in participants with dementia was significantly longer than those without dementia occurrence (all P<0.001).
Fig. 1.
Flow chart of study design
Table 1.
Baseline characteristics of study participants by incident dementia status
| Variables | Incident dementia | No incident dementia | P value |
|---|---|---|---|
| (N=4149) | (N=357945) | ||
| Age at baseline, years, mean (SD) | 64.49(4.53) | 56.87(8.00) | <0.001 |
| Sex, N (%) | <0.001 | ||
| Male | 2211(53.3%) | 159183(44.5%) | |
| Female | 1938(46.7%) | 198762(55.5%) | |
| Education, N (%) | <0.001 | ||
| ≥College or University degree | 903(21.8%) | 120477(33.7%) | |
| <College or University degree | 1831(44.1%) | 178522(49.9%) | |
| Skin color, N (%) | <0.001 | ||
| White | 3302(79.6%) | 273585(76.4%) | |
| Colored | 847(20.4%) | 84352(23.6%) | |
| Use of sun/UV protection, N (%) | <0.001 | ||
| No | 625(15.1%) | 33268(9.3%) | |
| Yes | 3477(83.8%) | 322624(90.1%) | |
| Do not go out in sunshine 3 | 45(1.1%) | 2038(5.7%) | |
| Employment status, N (%) | <0.001 | ||
| Yes | 753(18.1%) | 203004(56.7%) | |
| No | 3396(81.9%) | 154941(43.3%) | |
| Sleep duration, hours, N (%) | <0.001 | ||
| <7 | 1070(25.8%) | 87199(24.4%) | |
| 7 | 1246(30.0%) | 138878(38.8%) | |
| >7 | 1833(44.2%) | 131868(36.8%) | |
| PM2.5, ug/m3, mean (SD) | 10.04(1.05) | 9.97(1.05) | <0.001 |
| Fracture history, N (%) | 0.348 | ||
| Yes | 414(10.0%) | 34184(9.6%) | |
| No | 3711(90.0%) | 322213(90.4%) | |
| Vitamin D supplement, N (%) | 0.092 | ||
| Yes | 177(4.3%) | 13490(3.8%) | |
| No | 3944(95.7%) | 343441(96.2%) | |
| Hearing loss, N (%) | <0.001 | ||
| Yes | 1440(36.1%) | 90025(26.1%) | |
| No | 2548(63.9%) | 254597(73.9%) | |
| Smoking status, N (%) | <0.001 | ||
| Never | 1901 (46.1%) | 194259 (54.4%) | |
| Previous | 1805 (43.7%) | 126431 (35.4%) | |
| Current | 420 (10.2%) | 36256 (10.2%) | |
| Alcohol drinker status, N (%) | <0.001 | ||
| Never | 280 (6.8%) | 14058 (3.9) | |
| Previous | 295 (7.1%) | 12469 (3.5) | |
| Current | 3566 (86.1%) | 331202 (92.6) | |
| CVD diagnosed by doctor, N (%) | <0.001 | ||
| Heart disease a | 582(14.1%) | 16708(4.7%) | |
| Stroke | 243(5.9%) | 5503(1.5%) | |
| Hypertension | 1744(42.1%) | 98880(27.7%) | |
| TPA, MET-mins/week, mean (SD) | 2423.34 (2875.69) | 2449.20 (2746.68) | 0.555 |
| BMI, kg/m2, mean (SD) | 27.72(4.95) | 27.44(4.80) | <0.001 |
| Time spent in outdoor light, hours/day, mean (SD) | |||
| Average | 2.98(1.51) | 2.51(1.45) | <0.001 |
| Summer | 3.99(2.05) | 3.46(1.97) | <0.001 |
| Winter | 1.97(1.46) | 1.57(1.31) | <0.001 |
aHeart disease group includes myocardial infarction and angina. Data are n (%) and mean (SD). P values are derived using ether Student’s test, Mann-Whitney U test, or Chi-square test
Abbreviation: SD Standard deviation, UV Ultraviolet radiation, CVD Cardiovascular disease, TPA Total physical activity, BMI Body mass index
Association of time spent in outdoor light with dementia
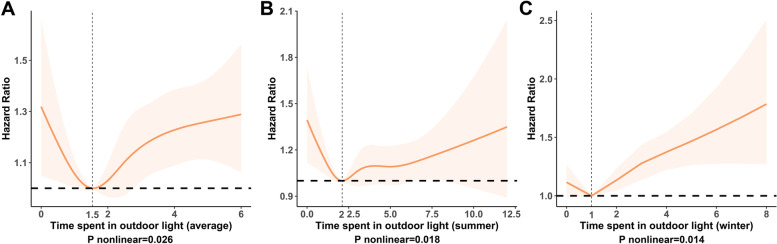
Estimated associations between sunlight exposure (as a continuous variable) and dementia outcome are shown from nonlinear spline models. Associations between time spent in outdoor light and risk of all-cause dementia were nonlinear in average, summer, and winter. Significant increases in risk were observed for dementia at both low and high exposure times (Fig. 2). The nadir for dementia risk was estimated from piecewise linear models to be at an average of 1.5 h/day, summer of 2 h/day, and winter of 1 h/day (Table 2). Multivariable Cox regression analyses showed that compared with participants who received an average of 1.5 h outdoor light per day, participants with less (adjusted HR=1.184 95%CI 1.000–1.401) or more (adjusted HR=1.210, 95%CI 1.054–1.390) time had a higher risk of dementia. Compared with participants who received 2 h outdoor light per day in summer, participants with less (adjusted HR=1.182, 95%CI 1.008–1.387) or more (adjusted HR=1.086, 95%CI 0.973–1.213) time tended to have a higher risk of dementia. This change point is 1 h for winter (less, adjusted HR=1.139, 95%CI 1.001–1.295; more, adjusted HR=1.242, 95%CI 1.129–1.367).
Fig. 2.
The correlation between outdoor light time and incident dementia during follow-up. The hazard ratio for dementia with the corresponding 95% confidence interval as a function of outdoor light time from Cox proportional hazard regression models adjusted for age, sex, education, skin color, use of sun/UV protection, employment status, sleep duration, PM2.5, fracture history, vitamin D supplement, hearing loss, smoking status, alcohol use, CVD, TPA, and BMI. Abbreviation: CVD cardiovascular disease, UV ultraviolet radiation, TPA total physical activity, BMI body mass index
Table 2.
Risk of incident dementia below and above the change point of sunlight exposure (categorical variable)
| Time spent in outdoor light | Number of incident dementia/Number of participants | Model 1 (Age, Sex) |
Model 2 (+Education, Skin color, Use of sun/UV Protection, Employment status, Sleep duration, PM2.5) |
Model 3 (+Fracture history, Vitamin D supplement, hearing loss, smoking status, alcohol use, CVD, TPA, BMI) |
|||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P Value | HR (95%CI) | P Value | HR (95%CI) | P Value | ||
| Average | |||||||
| 1.5 hours | 371/49938 | reference | reference | reference | |||
| below 1.5 hours | 535/72191 | 1.199 (1.050-1.369) | 0.007 | 1.176 (1.002-1.380) | 0.048 | 1.184 (1.000-1.401) | 0.049 |
| above 1.5 hours | 3243/239965 | 1.215 (1.091-1.353) | <0.001 | 1.204 (1.056-1.373) | 0.006 | 1.210 (1.054-1.390) | 0.007 |
| Summer | |||||||
| 2 hours | 657/82473 | reference | reference | reference | |||
| below 2 hours | 395/50233 | 1.213 (1.071-1.375) | 0.002 | 1.163 (0.998-1.354) | 0.053 | 1.182 (1.008-1.387) | 0.040 |
| above 2 hours | 3097/229388 | 1.116 (1.026-1.215) | 0.011 | 1.103 (0.994-1.224) | 0.065 | 1.086 (0.973-1.213) | 0.140 |
| Winter | |||||||
| 1 hours | 1099/126309 | reference | reference | reference | |||
| below 1 hours | 621/73877 | 1.169 (1.059-1.290) | 0.002 | 1.158 (1.025-1.308) | 0.018 | 1.139 (1.001-1.295) | 0.048 |
| above 1 hours | 2429/161908 | 1.235 (1.150-1.327) | <0.001 | 1.246 (1.139-1.363) | <0.001 | 1.242 (1.129-1.367) | <0.001 |
The results are derived from Cox hazard regression models in three models, with the sunlight exposure as a categorical variable. Bold indicates statistical significance (P value<0.05)
Abbreviation: HR Hazard ratio, CI Confidence interval, CVD Cardiovascular disease, TPA Total physical activity, BMI Body mass index
More specifically, we prefer to describe this nonlinear relationship with “J shape”. Since for sunlight exposure, a marked increase in risk was observed at low exposure, but a relatively slow elevation in risk at higher exposure (Fig. 2). Table 3 shows the results of the multivariate Cox proportional hazard regression models between sunlight exposure time and dementia as a continuous variable, which proves the above conclusion. Continuous exposure time was positively correlated with poor prognosis. Compared with the reference group (average, 1.5 h/day; summer, 2 h/day; winter, 1 h/day), both low and high sunlight exposure time groups were associated with a higher risk of dementia (average: low, HR=1.287 per 0.5 h, 95%CI 1.094–1.515 vs. high, HR=1.070 per 0.5 h, 95%CI 1.031–1.110); summer: low, HR=1.193 per 1 h, 95%CI 1.069–1.331 vs. high, HR=1.026 per 1 h, 95%CI 1.000–1.052; winter: low, HR=1.184 per 1 h, 95%CI 1.039–1.349 vs. high, HR=1.106 per 1 h, 95%CI 1.066–1.148).
Table 3.
Risk of incident dementia below and above the change point of sunlight exposure (continuous variable)
| Time spent in outdoor light | Model 1 (Age, Sex) |
Model 2 (+Education, Skin color, Use of sun/UV protection, Employment status, Sleep duration, PM2.5) |
Model 3 (+Fracture history, Vitamin D supplement, hearing loss, smoking status, alcohol use, CVD, TPA, BMI) |
|||
|---|---|---|---|---|---|---|
| HR (95%CI) | P Value | HR (95%CI) | P Value | HR (95%CI) | P Value | |
| Average | ||||||
| 1.5 hours | reference | reference | reference | |||
| below 1.5 hours (per 0.5 hour) | 1.353 (1.199-1.528) | <0.001 | 1.261 (1.084-1.468) | 0.003 | 1.287 (1.094-1.515) | 0.002 |
| above 1.5 hours (per 0.5 hour) | 1.071 (1.044-1.099) | <0.001 | 1.069 (1.033-1.105) | <0.001 | 1.070 (1.031-1.110) | <0.001 |
| Summer | ||||||
| 2 hours | reference | reference | reference | |||
| below 2 hours (per 1 hour) | 1.232 (1.137-1.336) | <0.001 | 1.180 (1.065-1.308) | 0.002 | 1.193 (1.069-1.331) | 0.002 |
| above 2 hours (per 1 hour) | 1.032 (1.013-1.051) | 0.001 | 1.029 (1.005-1.053) | 0.019 | 1.026 (1.000-1.052) | 0.048 |
| Winter | ||||||
| 1 hours | reference | reference | reference | |||
| below 1 hours (per 1 hour) | 1.182 (1.071-1.305) | 0.001 | 1.173 (1.038-1.326) | 0.010 | 1.184 (1.039-1.349) | 0.011 |
| above 1 hours (per 1 hour) | 1.099 (1.071-1.127) | <0.001 | 1.104 (1.066-1.142) | <0.001 | 1.106 (1.066-1.148) | <0.001 |
The results are derived from Cox hazard regression models in three models, with the sunlight exposure as a continuous variable. Bold indicates statistical significance (P value<0.05)
Abbreviation: HR Hazard ratio, CI Confidence interval, CVD Cardiovascular disease, TPA Total physical activity, BMI Body mass index
Additional analyses
Sensitivity analyses did not affect the assessment of the relationship between sunlight exposure time and dementia risk (Additional file 1). Similar results were observed after excluding participants with less than 3 and 10 years of follow-up, respectively (Additional file 1: Figure S2-3 and Table S3-4). The results of the White population showed the conclusion is also applicable to white participants (Additional file 1: Figure S1 and Table S2). To avoid the possible influence of missing data on the results, we repeated our analyses in a subset with complete covariates data (n=222479). The results were barely changed (Additional file 1: Figure S4 and Table S5).
Among participants equal to or older than 60 years old, there was a J-shaped relationship between sunlight exposure time and dementia risk, with the bottoms of the spline curve is about 2 h per day. This value is about 3 h/day in summer and about 1 h/day in winter. It is noteworthy that all these time points are longer than studies across all populations. A dose-dependent association between sunlight exposure time and the risk of dementia was not evident among participants under 60 (Additional file 1: Figure S5 and Table S6). The same phenomenon was observed in early and late onset dementia subgroups (Additional file 1: Figure S6 and Table S7). In females, the dementia risk was lowest at around an average of 2 h per day and increased dose-dependent if the time was below or above 2 h per day. A linear dose-response relationship was not observed between sunlight exposure time and the risk of dementia in males (Additional file 1: Figure S7 and Table S8). The subgroup analysis of sleep duration showed that even participants who slept 7 h per night needed sunlight to reduce the risk of dementia, with the bottoms of the spline curve being about 1.5 h per day. This value is 2 h/day in summer and 1 h/day in winter. (Additional file 1: Figure S8 and Table S9).
Discussion
The present study revealed that sunlight exposure time was associated with the risk of dementia. We observed a J-shaped association between outdoor sunlight exposure time and all-cause dementia risk, with the lowest risk at three change points (2 h/day in summer and 1 h/day in winter, and 1.5 h/day on average). This relationship did not change significantly after adjusting for various confounding factors.
Our findings were mostly consistent with previous evidence. A previous study has shown that a sunny June noon with an average sunshine time of about 105 min is most suitable for Alaska Anchorage to avoid vitamin D deficiency [24]. The latitude of this area (60°N) is like that of the UK. As we know, estimated cumulative UV exposure can be used to characterize an individual’s long-term vitamin D status [24]. Furthermore, the light and temperature models for the associations of light and temperature measurements with stroke subtypes also showed that stroke risk was the lowest in the second quartile of sunlight exposure, and higher or lower exposure increased the risk of stroke [25]. Surprisingly, the most appropriate sunlight exposure duration is shorter in winter than in summer. We speculate that this may be since some diseases are more likely to occur at low temperatures in winter. For example, as a seasonal disease, stroke showed an increased incidence in winter but a decreased incidence in summer [26, 27]. It is noteworthy that under less-than-ideal conditions, such as morning, evening, and cloudy conditions, there is less UV in the environment, and exposure to the same dose of UV may take a longer time.
There might be a variety of mechanisms underlying the relationship between sunlight and cognitive decline. Studies have pointed out that vitamin D receptors are widely present in neurons and glial cells. Vitamin D is involved in a variety of crucial pathways for brain health, involving neurotransmission, neuroprotection, regulation of immune response, inhibition of pro-inflammatory agents, and regulation of oxidative stress [2, 4]. There were evidences that the relationship between serum vitamin D concentration, and disease outcome does not seem to be a simple linear relationship [28, 29]. The data of long-term vitamin D levels in individuals are difficult to obtain. Since the progression of cognitive decline in the elderly is a long period, serum 25-hydroxyvitamin D, which is widely used to indicate vitamin D, can only indicate the temporary vitamin level. In addition, vitamin D levels in the tissues vary significantly with sunlight. Therefore, we used sunlight exposure time to roughly the vitamin D level in the elderly. Prospective studies are a trustworthy method to explore the association between vitamin D and cognitive function, but they take years or even decades. Most vitamin D is synthesized in the skin exposed to UV. The Department of Health and Social Care in the UK constantly rejects the proposal to add vitamin D into food, such as milk, bread, and orange juice [30]. Therefore, it is reasonable to use sunlight exposure to represent an individual’s long-term vitamin D level. In addition to vitamin D status, the environmental sunlight may also affect the physiological and cognitive function of the human body by regulating the circadian rhythm and affecting the suprachiasmatic nuclei (SCN) which are also called the body’s internal clock. One of the regulatory functions of SCN is to inhibit the pineal gland from converting serotonin into melatonin, which is involved in many mental and cognitive disorders [31].
However, excessive sunlight exposure is closely related to an increased risk of many negative consequences for human health, including sunburn, skin cancer (melanoma, lip cancer, and keratinocytes), and eye diseases (cataracts, ultraviolet keratitis). Suberythema UV doses also have biological effects. The absorption of UV by epidermal cells of the skin and eyes leads to the production of reactive oxygen species and nitrogen substances, which can injure biological molecules, such as the membrane lipids and DNA. UV directly damages DNA by forming pyrimidine dimers [32]. It is worth noting that in an upright state, the head of the human body is exposed directly to solar radiation, which may raise brain temperature to exceed core temperature, as broad-spectrum light penetrates several millimeters into the skin and heats the underlying tissues. There is an inverted U-shaped relationship between hyperthermia and cognitive performance. Significant cognitive declines in head and neck exposure tests are similar to that observed during hyperthermia therapy [33]. Long-term exposure of head and neck to sunlight causes a 1 °C increase in core temperature, which will cause worse cognitive performance [34]. Therefore, exploring the optimal sunlight exposure time can help us get more benefits and less damage.
The relationship between sunlight exposure and cognitive decline was more robust in females and older participants. The above gender tendency and age tendency are consistent with previous studies on the relationship between temperature and mortality, which found stronger associations in females and elders [35, 36]. Some reasons may explain our findings. Women are more likely to be affected by weather, and their clothes typically expose more skin. And elders are more likely to be affected by the changes during the aging process, such as cardiovascular events and the unignorable cumulative effect of light [37]. At the same time, the cumulative effect of light cannot be ignored. Besides, we found that elders seem to need a relatively longer duration of sunlight exposure to achieve the protective effect. The reason for this phenomenon may be that with increasing age, the amount of light reaching the retina decreases due to the yellowing of the lens and the contraction of the pupils. Although the compensation mechanism may maintain photosensitivity to some extent, yellowing is related to sleep disorders in the elderly [38, 39]. We found that even for participants who slept an optimal 7 h per night, sunlight exposure influenced the risk of dementia. Therefore, a 7-h sleep is not enough to offset the risk, and it needs to cooperate with the management of sunlight exposure.
We are the first to explore the nonlinear relationship between sunlight exposure time and the risk of dementia with a large cohort of cognitively intact participants. However, there are still some limitations. First, our research scheme reflects a potential sunlight mode. More details remain to be explored because the UK Biobank database did not collect the specific period of subjects receiving illumination. The use of rigorous design and detailed intervention characteristics reports, such as illuminance and temperature are essential for a prevention strategy. Second, differences in duration and UV intensity at different latitudes suggest that our conclusions may be hard to apply to all countries and regions. It needs further research on the specific optimal duration for other areas. Third, the duration of sunlight exposure is self-reported, which may lead to inaccurate responses, although most large-scale epidemiological studies rely on self-reported questionnaires. Fourth, bias due to unmeasured confounders persisted despite multiple corrections. Finally, the participants were predominantly white, with a slightly higher proportion of the affluent group. Further research are needed to investigate the extent to which these findings could be generalized to other populations.
Conclusions
In conclusion, there is a J-shaped correlation between outdoor sunlight exposure time and dementia risk. Detailed guidance on sunlight exposure can effectively prevent dementia in British people living in high latitudes far from the equator.
Supplementary Information
Additional file 1: Figure S1. The correlation between sunlight exposure time and incident dementia during follow-up in white participants. Figure S2. The correlation between sunlight exposure time and incident dementia during follow-up excluding participants less than three years of follow-up. Figure S3. The correlation between sunlight exposure time and incident dementia during follow-up excluding participants less than ten years of follow-up. Figure S4. The correlation between sunlight exposure time and incident dementia during follow-up in the subgroup with complete covariates data (n=222479). Figure S5. The correlation between sunlight exposure time and incident dementia during follow-up in age subgroups. Figure S6. The correlation between sunlight exposure time and incident dementia during follow-up in onset age subgroups. Figure S7. The correlation between sunlight exposure time and incident dementia during follow-up in sex subgroups. Figure S8. The correlation between sunlight exposure time and incident dementia during follow-up in sleep duration subgroups. Table S1. UKB field ID for dementia outcome used in the paper. Table S2. Risk of incident dementia below and above the change point of sunlight exposure in white participants. Table S3. Risk of incident dementia below and above the change point of sunlight exposure excluding participants less than three years of follow-up. Table S4. Risk of incident dementia below and above the change point of sunlight exposure excluding participants less than ten years of follow-up. Table S5. Risk of incident dementia below and above the change point of sunlight exposure in the subgroup with complete covariates data (n=222479). Table S6. Risk of incident dementia below and above the change point of sunlight exposure in subgroups of age. Table S7. Risk of incident dementia below and above the change point of sunlight exposure in subgroups of onset age. Table S8. Risk of incident dementia below and above the change point of sunlight exposure in sex subgroups. Table S9. Risk of incident dementia below and above the change point of sunlight exposure in subgroups of sleep duration.
Acknowledgements
The authors gratefully thank all the participants and professionals contributing to the UK Biobank.
Abbreviations
- UV
Ultraviolet radiation
- NIF
Non-image-forming
- BLT
Bright light treatment
- CVD
Cardiovascular disease
- TPA
Total physical activity
- BMI
Body mass index
- IPAQ
International Physical Activity Questionnaire
- ICD
International Classification of Diseases
- RCS
Restricted cubic spline
- HR
Hazard ratio
- CI
Confidence interval
- SCN
Superchiasmatic nuclei
Authors’ contributions
Conceptualization: JTY and LT. Data curation: JTY and WC. Formal analysis: LZM, YHM, YNO, SDC, and LY. Funding acquisition: JTY and WC. Investigation: LZM, YHM, YNO, SDC, and LY. Methodology: LZM, YHM, YNO, SDC, and LY. Project administration: JTY and WC. Resources: JTY and WC. Supervision: JTY and LT. Visualization: LZM and LY. Writing-original draft: LZM. Writing-review and editing: QD, WC, LT, and JTY. The authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82071201, 81971032), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), Research Start-up Fund of Huashan Hospital (2022QD002), Excellence 2025 Talent Cultivation Program at Fudan University (3030277001), and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Availability of data and materials
The data that support the findings of this study are available from the UK Biobank project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk.
Declarations
Ethics approval and consent to participate
All participants gave written informed consent prior to data collection. UK Biobank has full ethical approval from the NHS National Research Ethics Service (16/NW/0274).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lan Tan, Email: dr.tanlan@163.com.
Jin-Tai Yu, Email: jintai_yu@fudan.edu.cn.
References
- 1.Hjetland GJ, Pallesen S, Thun E, Kolberg E, Nordhus IH, Flo E. Light interventions and sleep, circadian, behavioral, and psychological disturbances in dementia: a systematic review of methods and outcomes. Sleep Med Rev. 2020;52:101310. doi: 10.1016/j.smrv.2020.101310. [DOI] [PubMed] [Google Scholar]
- 2.Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers Dement. 2014;10(3):296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 3.Eagles JM. The relationship between mood and daily hours of sunlight in rapid cycling bipolar illness. Biol Psychiatry. 1994;36(6):422–424. doi: 10.1016/0006-3223(94)91216-5. [DOI] [PubMed] [Google Scholar]
- 4.Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, Fried L, Kestenbaum BR, Kuller LH, Langa KM, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. doi: 10.1212/wnl.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–1015. doi: 10.1016/s1474-4422(18)30338-7. [DOI] [PubMed] [Google Scholar]
- 6.Videnovic A, Lazar AS, Barker RA, Overeem S. The clocks that time us'--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10(12):683–693. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetti F, Colombo C, Barbini B, Campori E, Smeraldi E. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62(3):221–223. doi: 10.1016/s0165-0327(00)00149-x. [DOI] [PubMed] [Google Scholar]
- 9.Kent ST, McClure LA, Crosson WL, Arnett DK, Wadley VG, Sathiakumar N. Effect of sunlight exposure on cognitive function among depressed and non-depressed participants: a REGARDS cross-sectional study. Environ Health. 2009;8:34. doi: 10.1186/1476-069x-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reusch J, Ackermann H, Badenhoop K. Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees N) population. Horm Metab Res. 2009;41(5):402–407. doi: 10.1055/s-0028-1128131. [DOI] [PubMed] [Google Scholar]
- 11.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye's important role in systemic health. Br J Ophthalmol. 2008;92(11):1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Health GB Dietary reference values for food energy and nutrients for the United Kingdom. Report of the panel on dietary reference values of the committee on medical aspects of food policy. Rep Health Soc Subj (Lond) 1991;41:1–210. [PubMed] [Google Scholar]
- 13.Department of Health GB Nutrition and bone health: with particular reference to calcium and vitamin D. Report of the Subgroup on Bone Health, Working Group on the Nutritional Status of the Population of the Committee on Medical Aspects of the Food Nutrition Policy. Rep Health Soc Subj (Lond) 1998;49:iii-xvii:1–24. [PubMed] [Google Scholar]
- 14.Farrar MD, Kift R, Felton SJ, Berry JL, Durkin MT, Allan D, Vail A, Webb AR, Rhodes LE. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am J Clin Nutr. 2011;94(5):1219–1224. doi: 10.3945/ajcn.111.019976. [DOI] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns AC, Saxena R, Vetter C, Phillips AJK, Lane JM, Cain SW. Time spent in outdoor light is associated with mood, sleep, and circadian rhythm-related outcomes: a cross-sectional and longitudinal study in over 400,000 UK Biobank participants. J Affect Disord. 2021;295:347–352. doi: 10.1016/j.jad.2021.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57(4):588–593. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 20.Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, Kivimäki M, Dugravot A, Singh-Manoux A. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. doi: 10.1038/s41467-021-22354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassett DR., Jr International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1396. doi: 10.1249/01.Mss.0000078923.96621.1d. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson T, Schnier C, Bush K, Rannikmäe K, Henshall DE, Lerpiniere C, Allen NE, Flaig R, Russ TC, Bathgate D, et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34(6):557–565. doi: 10.1007/s10654-019-00499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–953. doi: 10.1016/s2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrar MD, Webb AR, Kift R, Durkin MT, Allan D, Herbert A, Berry JL, Rhodes LE. Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: implications for targeted guidance on sun exposure. Am J Clin Nutr. 2013;97(6):1210–1216. doi: 10.3945/ajcn.112.052639. [DOI] [PubMed] [Google Scholar]
- 25.Kent ST, McClure LA, Judd SE, Howard VJ, Crosson WL, Al-Hamdan MZ, Wadley VG, Peace F, Kabagambe EK. Short- and long-term sunlight radiation and stroke incidence. Ann Neurol. 2013;73(1):32–37. doi: 10.1002/ana.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobel E, Zhang ZX, Alter M, Lai SM, Davanipour Z, Friday G, McCoy R, Isack T, Levitt L. Stroke in the Lehigh Valley: seasonal variation in incidence rates. Stroke. 1987;18(1):38–42. doi: 10.1161/01.str.18.1.38. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Levi CR, Attia JR, D'Este CA, Spratt N, Fisher J. Seasonal variation in stroke in the Hunter Region, Australia: a 5-year hospital-based study, 1995-2000. Stroke. 2003;34(5):1144–1150. doi: 10.1161/01.Str.0000067703.71251.B6. [DOI] [PubMed] [Google Scholar]
- 28.Geng T, Lu Q, Wan Z, Guo J, Liu L, Pan A, Liu G. Association of serum 25-hydroxyvitamin D concentrations with risk of dementia among individuals with type 2 diabetes: a cohort study in the UK Biobank. PLoS Med. 2022;19(1):e1003906. doi: 10.1371/journal.pmed.1003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou A, Selvanayagam JB, Hyppönen E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur Heart J. 2021. 10.1093/eurheartj/ehab809. [DOI] [PubMed]
- 30.Xin S, Luan D, Wang X, Wang F, Liu Y, Gao Q. Relationship between cumulative ultraviolet exposure and cognitive function in a rural elderly Chinese population. Int J Geriatr Psychiatry. 2018;33(8):1121–1126. doi: 10.1002/gps.4902. [DOI] [PubMed] [Google Scholar]
- 31.Kim SY, Bang M, Wee JH, Min C, Yoo DM, Han SM, Kim S, Choi HG. Short- and long-term exposure to air pollution and lack of sunlight are associated with an increased risk of depression: a nested case-control study using meteorological data and national sample cohort data. Sci Total Environ. 2021;757:143960. doi: 10.1016/j.scitotenv.2020.143960. [DOI] [PubMed] [Google Scholar]
- 32.Fleury N, Geldenhuys S, Gorman S. Sun exposure and its effects on human health: mechanisms through which sun exposure could reduce the risk of developing obesity and cardiometabolic dysfunction. Int J Environ Res Public Health. 2016;13(10). 10.3390/ijerph13100999. [DOI] [PMC free article] [PubMed]
- 33.Schmit C, Hausswirth C, Le Meur Y, Duffield R. Cognitive functioning and heat strain: performance responses and protective strategies. Sports Med. 2017;47(7):1289–1302. doi: 10.1007/s40279-016-0657-z. [DOI] [PubMed] [Google Scholar]
- 34.Piil JF, Christiansen L, Morris NB, Mikkelsen CJ, Ioannou LG, Flouris AD, Lundbye-Jensen J, Nybo L. Direct exposure of the head to solar heat radiation impairs motor-cognitive performance. Sci Rep. 2020;10(1):7812. doi: 10.1038/s41598-020-64768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller MC, Fredrickson BL, Ybarra O, Côté S, Johnson K, Mikels J, Conway A, Wager T. A warm heart and a clear head. The contingent effects of weather on mood and cognition. Psychol Sci. 2005;16(9):724–731. doi: 10.1111/j.1467-9280.2005.01602.x. [DOI] [PubMed] [Google Scholar]
- 36.Graham SE, McCurdy T. Developing meaningful cohorts for human exposure models. J Expo Anal Environ Epidemiol. 2004;14(1):23–43. doi: 10.1038/sj.jea.7500293. [DOI] [PubMed] [Google Scholar]
- 37.Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev. 2002;24(2):190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- 38.La Morgia C, Ross-Cisneros FN, Sadun AA, Carelli V. Retinal Ganglion cells and circadian rhythms in Alzheimer's disease, parkinson's disease, and beyond. Front Neurol. 2017;8:162. doi: 10.3389/fneur.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najjar RP, Chiquet C, Teikari P, Cornut PL, Claustrat B, Denis P, Cooper HM, Gronfier C. Aging of non-visual spectral sensitivity to light in humans: compensatory mechanisms? PLoS One. 2014;9(1):e85837. doi: 10.1371/journal.pone.0085837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The correlation between sunlight exposure time and incident dementia during follow-up in white participants. Figure S2. The correlation between sunlight exposure time and incident dementia during follow-up excluding participants less than three years of follow-up. Figure S3. The correlation between sunlight exposure time and incident dementia during follow-up excluding participants less than ten years of follow-up. Figure S4. The correlation between sunlight exposure time and incident dementia during follow-up in the subgroup with complete covariates data (n=222479). Figure S5. The correlation between sunlight exposure time and incident dementia during follow-up in age subgroups. Figure S6. The correlation between sunlight exposure time and incident dementia during follow-up in onset age subgroups. Figure S7. The correlation between sunlight exposure time and incident dementia during follow-up in sex subgroups. Figure S8. The correlation between sunlight exposure time and incident dementia during follow-up in sleep duration subgroups. Table S1. UKB field ID for dementia outcome used in the paper. Table S2. Risk of incident dementia below and above the change point of sunlight exposure in white participants. Table S3. Risk of incident dementia below and above the change point of sunlight exposure excluding participants less than three years of follow-up. Table S4. Risk of incident dementia below and above the change point of sunlight exposure excluding participants less than ten years of follow-up. Table S5. Risk of incident dementia below and above the change point of sunlight exposure in the subgroup with complete covariates data (n=222479). Table S6. Risk of incident dementia below and above the change point of sunlight exposure in subgroups of age. Table S7. Risk of incident dementia below and above the change point of sunlight exposure in subgroups of onset age. Table S8. Risk of incident dementia below and above the change point of sunlight exposure in sex subgroups. Table S9. Risk of incident dementia below and above the change point of sunlight exposure in subgroups of sleep duration.
Data Availability Statement
The data that support the findings of this study are available from the UK Biobank project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk.