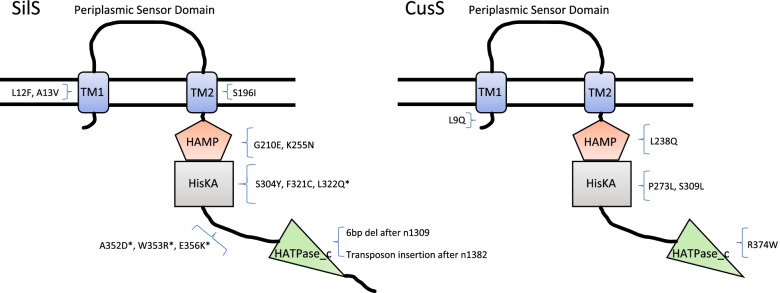
Fig. 3.
Schematic diagram of sensor histidine kinase SilS and CusS showing location of all mutations found in K. pneumoniae strains generated from adaptation experiments, in relation to domain structure. TM1, Transmembrane region 1 (12–34 SilS; 15–34 CusS); TM2, Transmembrane region 2 (188–207 SilS; 184–206 CusS); HAMP, putative regulator of phosphorylation (208–261 SilS; 204–257 CusS)); HisKA, dimerization/histidine phosphotransfer (262–328 SilS; 258–324 CusS); HATPase_c, ATP binding domain (372–483 SilS; 368–477 CusS). * indicates that this mutation was observed in more than one strain