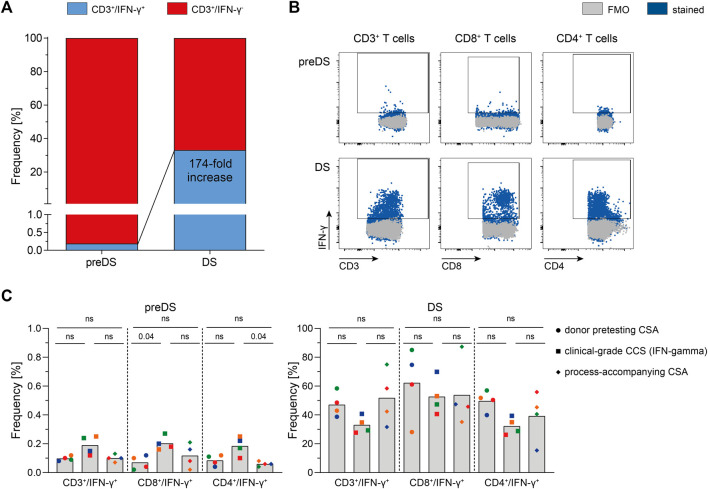
FIGURE 2.
Clinical manufacturing of SARS-CoV-2-specific T cells (n = 4). SARS-CoV-2-specific T cells were magnetically enriched under GMP-compliant conditions using Cytokine Capture System (CCS) and CliniMACS Prodigy and analyzed via flow cytometry. (A) Enrichment of SARS-CoV-2-specific Interferon-gamma (IFN-γ)-secreting T cells using CCS and CliniMACS Prodigy. Data are shown as mean of data obtained from n = 4 manufacturing processes. (B) Representative FACS plots depicting IFN-γ production in indicated T cell subsets of in-process control (pre-enrichment; preDS) and the magnetically enriched T cell product (drug substance, DS). (C) Summarized results of clinical-grade manufacturing in comparison to corresponding donor pretesting Cytokine Secretion Assay (CSA) and process-accompanying CSA. Bars represent mean, each symbol represents data obtained from one donor (same colors indicate matched data), n = 4 convalescent COVID-19 patients. Statistical analysis was performed using Friedman Test, followed by Dunn’s multiple comparison; ns not significant.