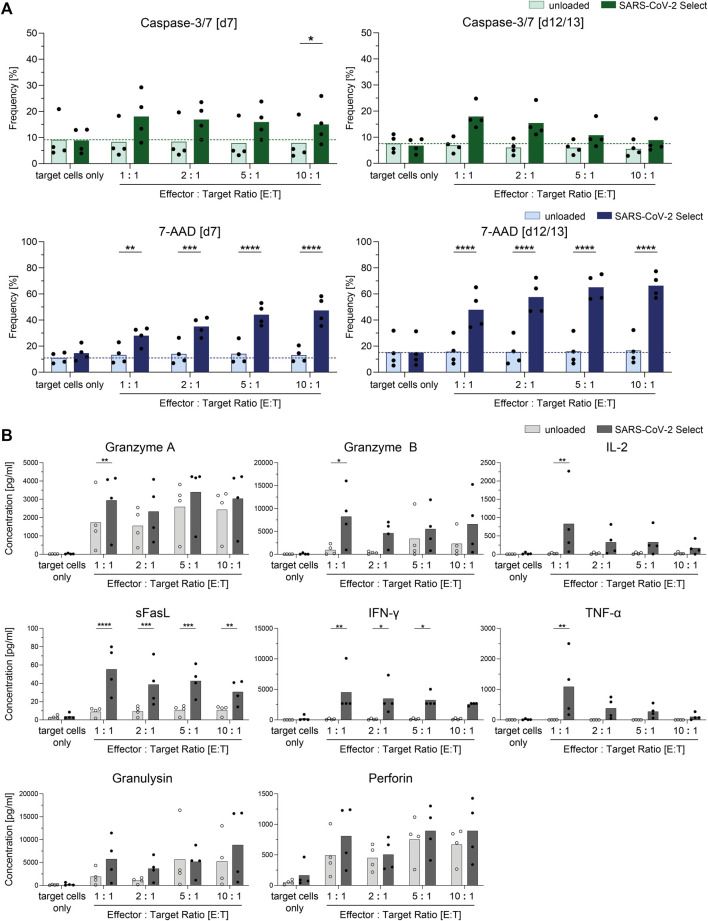
FIGURE 4.
Cytotoxic potential of clinical-grade SARS-CoV-2-specific T cells (n = 4). SARS-CoV-2-specific T cells obtained by Cytokine Capture System (CCS) and CliniMACS Prodigy were expanded for 7–13 days and subjected to cytotoxicity assays using unloaded and SARS-CoV-2 Select-loaded autologous PBMCs as target cells. (A) Frequencies of cells with active caspase-3/7 or 7-AAD+ cells among target cells (CellTrace Proliferation dye positive). Light bars: unloaded target cells. Dark bars: loaded target cells. Results are displayed as individual results and as means. (B) Cell culture supernatants from cytotoxicity assays from day 12/13 were analyzed with respect to presence of cytotoxic effector molecules by LEGENDPlex Assay. Results are displayed as individual results and as means, n = 4 manufacturing processes. Statistical significance was calculated using Two-way ANOVA and Sidak’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.