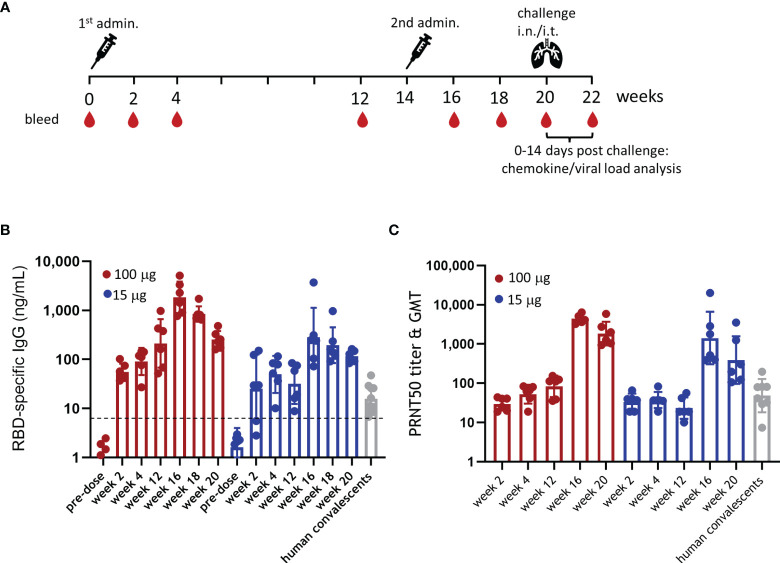
Figure 1.
Experimental design and immunogenicity of ABNCoV2. (A) Schematic study design with non-human primates (NHP) (N = 6 per group) vaccinated intramuscularly with 100 or 15 μg ABNCoV2 in weeks 0 and 14 and challenged with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in week 20. Four (4) additional non-vaccinated NHP were added as controls. At regular intervals as indicated, animals were bled. (B) RBD-specific IgG was measured by ELISA, and (C) SARS-CoV-2 neutralizing antibodies were assessed by PRNT. For comparison, 10 human plasma samples positive for SARS-CoV-2 antibodies (human convalescents, gray symbols) were analyzed as well. Filled circles represent individual values and columns depict the geometric mean (ELISA, B) or geometric mean titers (PRNT, C) of the group +/geometric standard deviation. The horizontal dotted line in (B) represents the mean + 2 times the standard deviation of 50 untreated naive NHP to indicate background responses.