Figure 4.
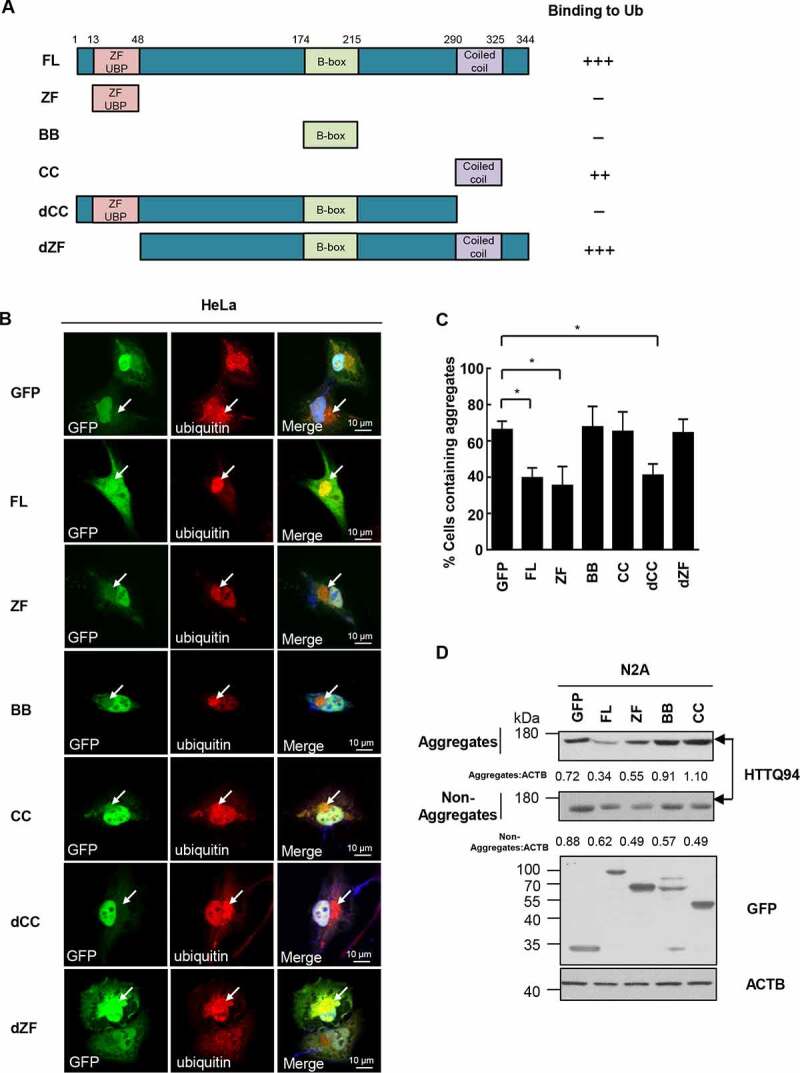
CC domain binds aggregates and ZF domain is required for aggregate clearance. (a) The TRIM44 constructs employed were GFP-tagged wild-type (WT) TRIM44; full-length (FL) TRIM44 (amino acids 1 to 344); ZF domain, a construct amino acids 13 to 48; BB domain, a construct amino acids 174 to 215; CC domain, a construct amino acids 290 to 325; dCC domain, a construct missing the CC domain (amino acids 1 to 290); and dZF domain, a construct missing the ZF domain (amino acids 48 to 344). (b) Shown are laser scanning confocal microscopy images of immunofluorescence staining for exogenous GFP-TRIM44 truncates (green) and Ubiquitin (red) with proteasomal inhibitor MG132 (5 µM) for 24 h. Cells were incubated with merged images with overlapping immunoreactivity are shown in yellow. All experiments were replicated three independent times with similar results. Scale bars: 10 μm. (c) Relative percentage of cells with aggregates (means + SD, n = 3) (*, P < 0.05). (d) Steady-state levels of HTTQ94 expressed in N2A cells transfected with GFP-tagged TRIM44 truncates (full-length, ZF domain, BB domain and CC domain), analyzed by western blots. Non-Aggregates: NP-40-soluble; Aggregates: SDS-soluble, NP40 insoluble. Relative ratios of non-aggregates or aggregates versus ACTB are indicated.
