Fig. 1.
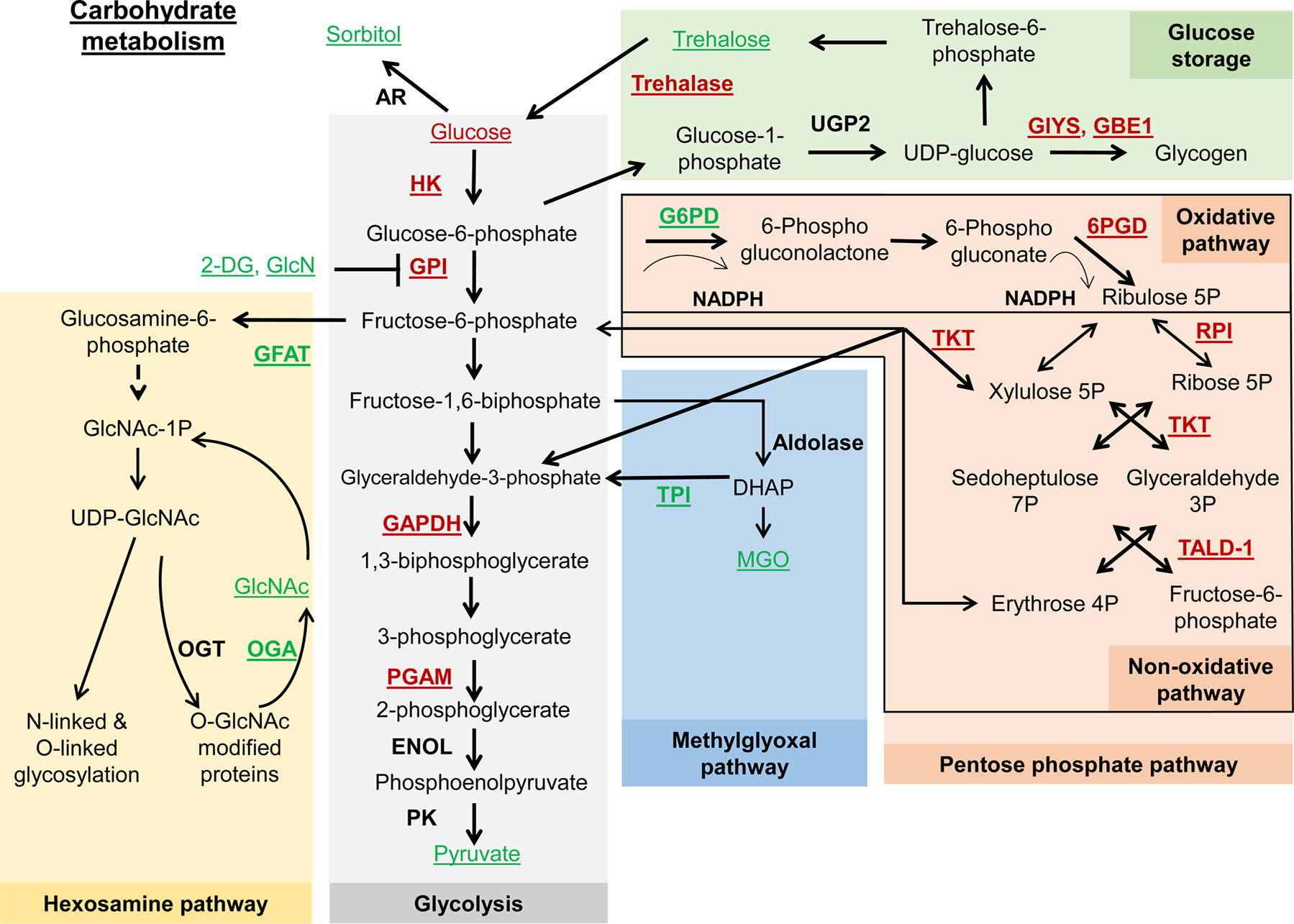
Schematic representation of glycolysis and related metabolic pathways. Underlined are metabolites and enzymes that were associated with lifespan extension. Red font color represents downregulation or depletion from food, while green font color represents overexpression or supplementation. Dashed line represents that multiple steps are involved. In the glycolysis pathway, glucose is broken down into pyruvate, producing ATP. Lifespan extension was associated with glycolysis inhibition through the downregulation of hexokinase (HK), glucose isomerase (GPI), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), or phosphoglycerate mutase (PGAM); or through addition of inhibitors such as 2-Deoxyglycose (2-DG) and D-glucosamine (GlcN). The hexosamine pathway converts fructose-6-phosphate to UDP-N-acetylglucosamine (UDP-GlcNAc). Lifespan extension was associated with an increased expression of glutamine-fructose 6-phosphate aminotransferase (GFAT) and O-GlcNAcase (OGA), as well as with added acetylglucosamine (GlcNAc). The methylglyoxal pathway produces methylglyoxal (MGO) from glyceraldehyde-3-phosphate and dihydroxyacetone phosphate (DHAP). While excessive MGO can disrupt protein function, moderate supplementation was associated with increased lifespan in worms. Increased expression of triosephosphate isomerase (TPI) also increased lifespan. The pentose phosphate pathway (PPP) consists of the oxidative and nonoxidative branches. Lifespan extension was associated with downregulation of 6-phosphogluconate dehydrogenase (6PGD), ribose-5-phosphate isomerase (RPI), transketolase (TKT), and transaldolase (TALD-1), as well as with upregulation of glucose-6-phosphate dehydrogenase (G6PD). Downregulation of enzymes responsible for glycogen synthesis (glycogen synthase, GlyS; and 1,4-alpha-glucan branching enzyme 1, GBE1) extended lifespan. Increased levels of trehalose and downregulation of trehalase was associated with extended lifespan in worms.
