Fig. 2.
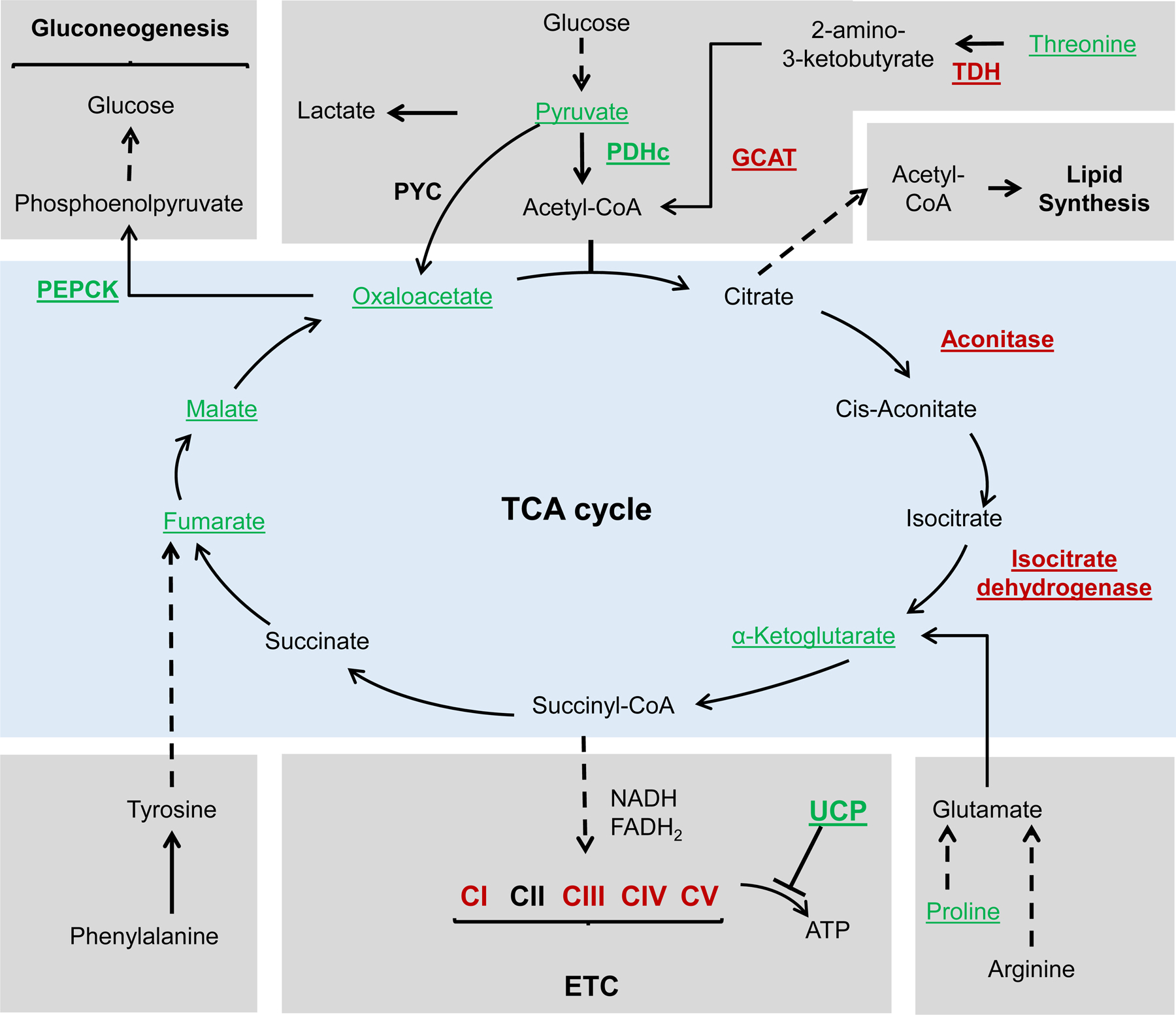
Schematic representation of the TCA cycle and related metabolic pathways. Underlined are metabolites and enzymes that were associated with lifespan extension. Red font color represents downregulation or depletion from food, while green font color represents overexpression or supplementation. Dashed line represents that multiple steps are involved. Before entering the TCA cycle, pyruvate must be converted to acetyl-CoA through the pyruvate dehydrogenase complex (PDHc). Overexpression of the dihydrolipoamide acetyltransferase (E2 component) of the PDHc extended lifespan in yeast. In addition, downregulation of pyruvate dehydrogenase kinase (not shown), an inhibitor of PDHc, extended lifespan in worms. Threonine can be converted to acetyl-CoA through a series of reactions. Threonine supplementation, as well as downregulation of the enzymes l-threonine-3-dehydrogenase (TDH) and glycine-C-acetyltransferase (GCAT), extended lifespan in yeast. Dietary supplementation of several TCA cycle intermediates, including oxaloacetate, α-ketoglutarate, fumarate, and malate, was associated with lifespan extension. Downregulation of aconitase and isocitrate dehydrogenase, two enzymes in the TCA cycle, also extended lifespan. The electron transport chain (ETC) is a series of complexes which ultimately generates ATP through electron transfer and redox reactions. Downregulation of components of complexes I, II, IV and V was associated with lifespan extension in worms. In addition, lifespan was extended through the expression of some mitochondrial uncoupling proteins (UCPs). Gluconeogenesis is a process that allows cells to convert TCA intermediates into glucose under nutrient starvation. Phosphoenolpyruvate carboxykinase (PEPCK) is a key enzyme in this process, and overexpression of PEPCK extends lifespan in worms.
