A previously healthy 6-month boy was admitted in the emergency room of a peripheral hospital for recurrent seizures after an episode of fever. He was born full-term to non-consanguineous Caucasian parents by cesarean section because of premature rupture of membranes. APGAR scores were 9 at 1 and 5 min, respectively. Until the acute episode, his neurological and behavioral development was appropriate for age. At 6 months, he presented an episode of acute onset fever treated at home by general physician with oral antibiotic and antipyretic, lasting 1 day only. After 3 days, the child experienced a first non-febrile focal onset motor seizure (right head and gaze deviation, hand automatisms), lasting 1–2 min, at home. Subsequently, the child was admitted in the emergency room of a peripheral hospital where he experienced a new and more severe episode characterized by right head and gaze deviation, increased muscle tone, hand automatisms, perioral cyanosis, lasting about 1–2 min, and treated by ER physician with intramuscular midazolam (at dosage of 0.2 mg/kg).
At admission to our pediatric infectious disease unit, a nasopharyngeal swab for the SARS-CoV-2 virus via reverse transcriptase-polymerase chain reaction (RT-PCR) resulted positive for the B.1.1.7 variant of SARS-CoV-2, also known as VOC 202,012/01.
Neurological examination revealed focal signs including persistent left conjugate eye deviation, mild hypotonia, and reduced deep tendon reflexes, in the absence of meningeal signs and abnormal level of consciousness.
Physical examination showed regular hydration and cardio-respiratory activity. Patient blood tests showed normal blood count and slightly increased D-dimer (1.32; [normal < 0.6]) without evidence of systemic inflammatory response (C-reactive protein, procalcitonin and fibrinogen). Chest X-ray and echocardiogram did not show abnormalities. Brain CT scan revealed cortico-subcortical hypodense areas in the left frontal and parietal lobes (Fig. 1 A). In order to better define the etiology of brain damage and neurological signs, a lumbar puncture was performed 2 days after the brain CT scan. Cerebrospinal fluid (CSF) was clear with normal protein concentration (25 mg/dL range 15–45 mg/dL), glucose level (48 mg/dL range, 40–80 mg/dL), and leukocyte count (2 cells/μL (range, < 10 n/μL). PCRs for SARS-CoV-2 RNA, herpes virus (HSV 1–2, herpes 6A-B, parvovirus, CMV, EBV), and bacterial cultures in CSF were negative.
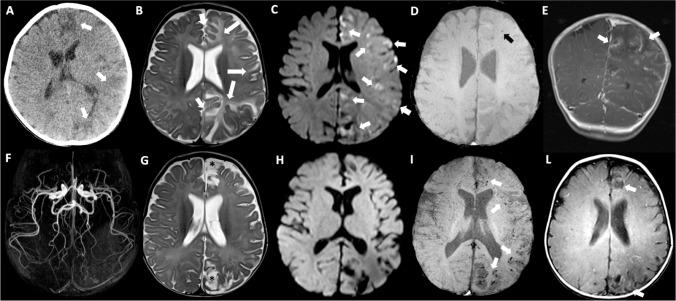
Fig. 1.
Brain CT executed at the onset of seizures showed cortico-subcortical hypodense areas in left frontal and parietal lobes (arrows in A). Brain MRI obtained after two days from CT (B,C,D,E) showing involvement of the left hemisphere especially affecting frontal, parietal and cingulate regions with little mass effect. Note the slight hyperintensity of the affected cortex compared to the controlateral normal cortex (short arrows), and the more marked subcortical hyperintensity related to vasogenic edema on T2-weighted image (long arrows) (B). Restricted diffusion is evident on DWI in a lot of cortico-subcotical regions on the left side (arrows in C). Only one punctiform hypointense image is observable in the white matter of the left frontal lobe on SWI (black arrow in D). Cortical and leptomeningeal enhancing streaks (arrows) are quite evident after gadolinium injection on coronal T1-weighted image (E). MRI of the brain at two weeks follow-up (F, G,H,I,L). 3D TOF MR Angiography MIP image of the circle of Willis shows no vascular stenoses (F). Gliotic-malacic evolution of some previously affected areas is especially observable in left parasagittal frontal and parietal lobes (asterisks) in association with ventricular and ipsilateral sulcar enlargement on axial T2-weighted image (G). Cytotoxic edema and signal restriction disappeared on DWI (H). Punctiform cortico-subcortical microbleeds, and curvilinear hyperintensities related to cortical laminar necrosis, on SWI (arrows in I), and T1-weighted images without gadolinium injection (arrows in L) in the left affected areas
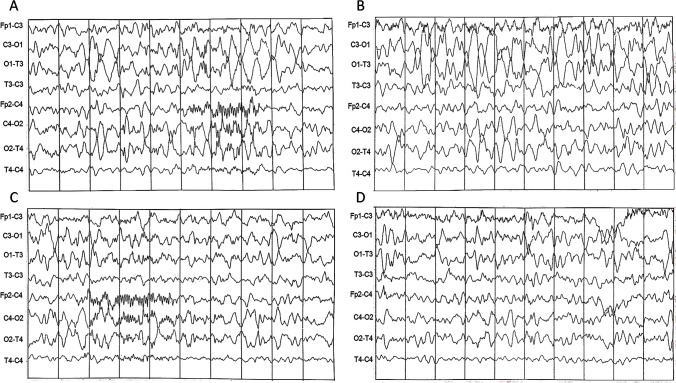
A sleep electroencephalography showed epileptic abnormalities on the left frontal leads with a slowing of background activity on the left posterior areas (Fig. 2 A–D). Phenobarbital (3.3 mg/kg/day) was started.
Fig. 2.
Sleep EEG showing an asymmetry of cerebral background activity with high voltage delta activity on the left temporo-occipital areas and reduced representation of spindles on the left frontal areas (A–C). Spike-waves complexes were also evident on the left frontal regions (D). EEG parameters: Notch 50 Hz, LF: 30 Hz, TC: 0,1 sec
Six days after symptom onset, brain magnetic resonance imaging (MRI) revealed cortical and subcortical T2-hyperintensity related to vasogenic edema in the frontal, parietal, and cingulate regions of the left hemisphere with slight mass effect (Fig. 1 B–E).
Other potential viral pathogens, including coronavirus HCoV-NL63, HCoV-OC43, HCoV-229E, HKU1, RSV A-B, rhinovirus, metapneumovirus, influenza A-B, adenovirus, bocavirus, parechovirus, and enterovirus, were also ruled out by PCRs.
Screening for metabolism inborn errors (homocysteine, acylcarnitine, aminoacidemia, urinary organic acids, lactic acidemia, ammonia, blood gas analysis) and first-line immunological evaluation (total immunoglobulin, lymphocyte subpopulations) did not provide significant results.
To investigate a potential autoimmune mechanism involved in encephalitis, an extensive autoantibody panel, including antibodies against LG1, CASPR2, AMPA 1–2, NMDA, GABAb1, DPPX, AQP4, MOG, VOKCs, and GLUR3, was performed in blood and CSF, with normal or negative results.
On the basis of clinical signs and neuroimages, SARS-CoV2 encephalitis resulting in a unilateral cerebral vasculitis was diagnosed. After 2 weeks from admission, the patient was treated with 20 mg/kg/day intravenous methylprednisolone for three days followed by oral prednisolone 1.5 mg/kg/day for 2 months.
Because of a hypercoagulability (increased levels of coagulation factors V, VIII, XII), a prophylactic treatment with low molecular weight heparin (LMWH) at 80 mg/kg/dose was administered subcutaneously twice a day for 2 weeks. During the following days, the general clinical conditions progressively improved and all abnormal neurological focal signs observed at admission progressively but rapidly disappeared, with full remission in approximately 3 weeks.
As shown in Fig. 1 (F–L), a repeat brain MRI, obtained 2 weeks later, showed a gliotic-malacic evolution of some of the previously affected areas, especially in the left parasagittal frontal and parietal lobes (on T2), and the appearance of punctiform cortico-subcortical microbleeds and curvilinear hyperintensities related to laminar necrosis of the cortex (on SWI and T1). MRI angiography of the circle of Willis showed no vascular stenosis. The localization and evolution of brain lesions and the negativity of the laboratory reasonably ruled out differential diagnoses including bacterial/viral meningoencephalitis, ischemic stroke, metabolic diseases such as MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes) syndrome, and primary immunodeficiencies. There were no criteria fo multisystem inflammatory syndrome in children (MIS-C).
A third brain MRI, performed 1 month after the onset of symptoms, showed stabilization of mono-hemispheric brain lesions. The first negative RT‐PCR test for SARS-CoV-2 was obtained 36 days after the first positive swab test.
Neurological and development examination 10 months after the acute episode was normal. He was seizure-free under phenobarbital. A sleep EEG showed rare epileptic abnormalities on the left posterior leads. Visual- and auditory-evoked potentials were also normal. Coagulation parameters returned to normal values. To rule out possible genetic predisposition to early-onset seizures, a whole-exome sequencing was performed and revealed a missense variant NM_014191.4:c.5515G > A, p.Gly1839Arg in the SCN8A gene, interpreted of unknown significance by Agilent Alissa Interpret prediction tool and according to American College Medical Genetics variant classification guidelines. This variant was also inherited by the father who does not have history of seizure or epilepsy.
To date, a wide variety of neurological symptoms and conditions have been described in patients with SARS-CoV-2 infection. The most frequent neurological symptoms described in adults include headache, dizziness, myalgia, anosmia, and dysgeusia. More severe cases showed hemorrhagic encephalopathy, stroke, Guillain–Barré syndrome, ADEM, anti-MOG-encephalitis, and inflammatory vasculopathy [1].
Recently, post-mortem neuroimaging studies in adult patients with COVID-19 revealed diffuse petechial hemorrhages at the gray-white matter junction of the neocortex and neuropathological studies highlighted the presence of vascular intramural inflammatory infiltrates, consistent with SARS-CoV-2-associated endotheliitis [2].
Neurological manifestations are rare in children with COVID-19. However, those presenting with multisystem inflammatory syndrome (MIS-C) are at high risk for neurological complications, including acute seizures, headaches, positive meningeal signs, and altered mental status.
We describe for the first time an infant affecting by a mono-hemispheric encephalitis triggered by SARS-CoV-2. The exact role of the virus in the brain damage pathogenesis is not clearly understood; in particular, a direct or indirect pathogenic role of the virus might be hypothesized.
In a recent systematic review on patients with SARS-COv2-related neurological symptoms, the viral RNA has been detected in the CSF only in 6% of cases, suggesting that mechanisms other than viral replication in the CNS contribute to neuropathology [3].
In this case, the clinical and neuroradiological features may be consistent with a virus-triggered unilateral vascular inflammation (vasculitis). Vasculitis of the CNS is a brain inflammatory condition, due to different etiologies, including infections, systemic inflammatory diseases, drugs, malignancy, or—alternatively—it may be idiopathic, as in the childhood primary angiitis of the CNS. Infections are the most common etiology of secondary vasculitis. For example, varicella zoster virus may trigger a CNS vasculitis, often labelled as post-varicella angiopathy. The identification and rapid management of the underlying condition are crucial in controlling secondary CNS vasculitis. In para-or -post-infectious CNS vasculitis, in addition to anti-microbial or anti-viral therapy, it is important to target systemic inflammation and immune dysregulation, using immunosuppressive therapies as steroids or intravenous immunoglobulin (IVIg) [4].
Another important peculiarity of this case is the exclusive unilateral cerebral involvement that is reported only in rare autoimmune encephalitis such as the Rasmussen syndrome, a rare neurological disease, characterized by progressive atrophy of the unilateral hemisphere and clinically by epilepsia partialis continua, invariably hemiparesis, and cognitive impairment. Interestingly, one of the pathogenic hypotheses to explain the mono-hemispheric involvement in the Rasmussen encephalitis is the slow progression of a viral infection and immunotherapy; in particular, steroids or IVIg is one of the first-line treatments in addition to anti-epileptic drugs [5].
A slight alteration of coagulation parameters is another clinical clue present in our patient. Coagulopathy is a common feature of severe COVID-19. To date, current evidence suggests that COVID-19-associated coagulopathy is a combination of low-grade disseminated intravascular coagulation and pulmonary thrombotic microangiopathy, which could have a significant impact on organ dysfunction in most patients with severe disease. We do not rule out that hypercoagulability in association with COVID-19-induced cerebral endotheliopathy may contribute to microcirculatory cerebral alterations and subsequent brain damage present in this case.
In conclusion, this case highlights the importance and severity of neurological manifestations in children with COVID-19. Although rare, they may have a broad clinical presentation including focal severe rapidly progressive brain damage. The clinical course may rapidly improve, and potentially, the long-term consequences may be reduced if inflammation is promptly controlled with immunotherapies. However, a strict neurological and EEG follow-up will be mandatory in order to promptly identify neurological deficiency and the onset of focal seizures, secondary to structural brain damage.
Author contribution
Dr. Terrone, Prof. Guarino, and Dr. Nunziata conceptualized and designed the study and reviewed and revised the manuscript. Dr. Alessio, Dr. Vassallo, Dr. Romano A, Dr. Lo Vecchio, Dr. D’Amico, Dr. Buono, and Dr. Bruzzese collected data, drafted the initial manuscript, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
None.
Informed consent
Patient data were anonymous, and diagnostic therapeutic interventions fulfil international guidelines criteria.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/13/2022
A correct tagging of the Author Andrea Lo Vecchio has been updated.
References
- 1.Lindan CE, Mankad K, Ram D, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5:167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirschenbaum D, Imbach LL, Rushing EJ, et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol Appl Neurobiol. 2021;47:454–459. doi: 10.1111/nan.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis A, Frontera J, Placantonakis DG, et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. 2021;421:117316. doi: 10.1016/j.jns.2021.117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twilt M, Benseler SM. Treatment of CNS vasculitis in children. Curr Treat Options in Rheum. 2015;1:365–380. doi: 10.1007/s40674-015-0032-5. [DOI] [Google Scholar]
- 5.Tang C, Luan G, Li T. Rasmussen’s encephalitis: mechanisms update and potential therapy target. Ther Adv Chronic Dis. 2020;11:2040622320971413. doi: 10.1177/2040622320971413. [DOI] [PMC free article] [PubMed] [Google Scholar]